Polymer with anti-cancer property and preparation method and application of polymer
A polymer and performance technology, applied in the field of biomedical engineering materials, can solve problems such as consumption, and achieve the effect of simple material composition, convenient operation, and mild preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Synthesis of N-aminoethylpiperazine-terminated hyperbranched polyamidoamines (PAAs)
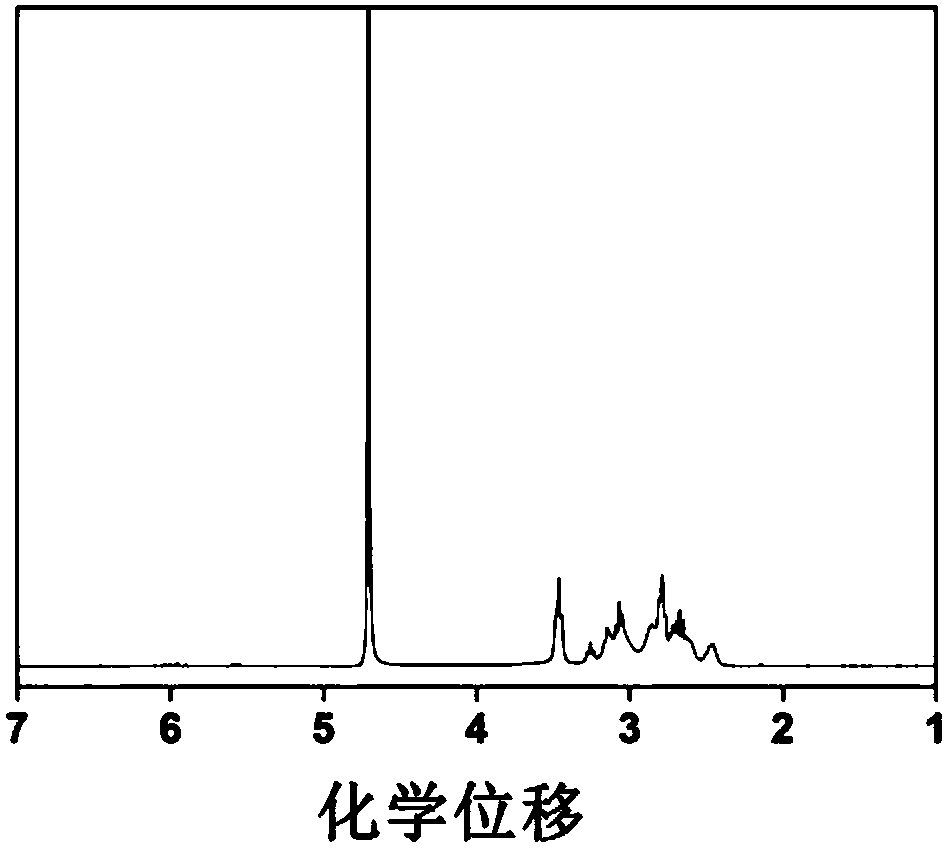
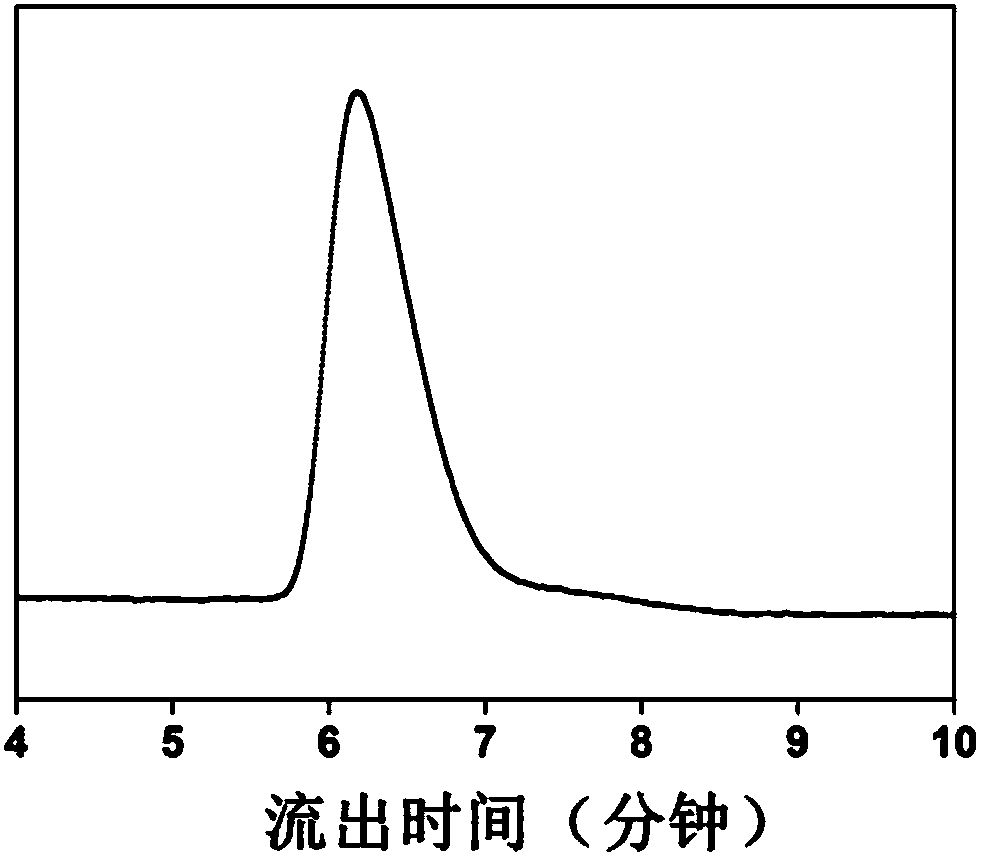
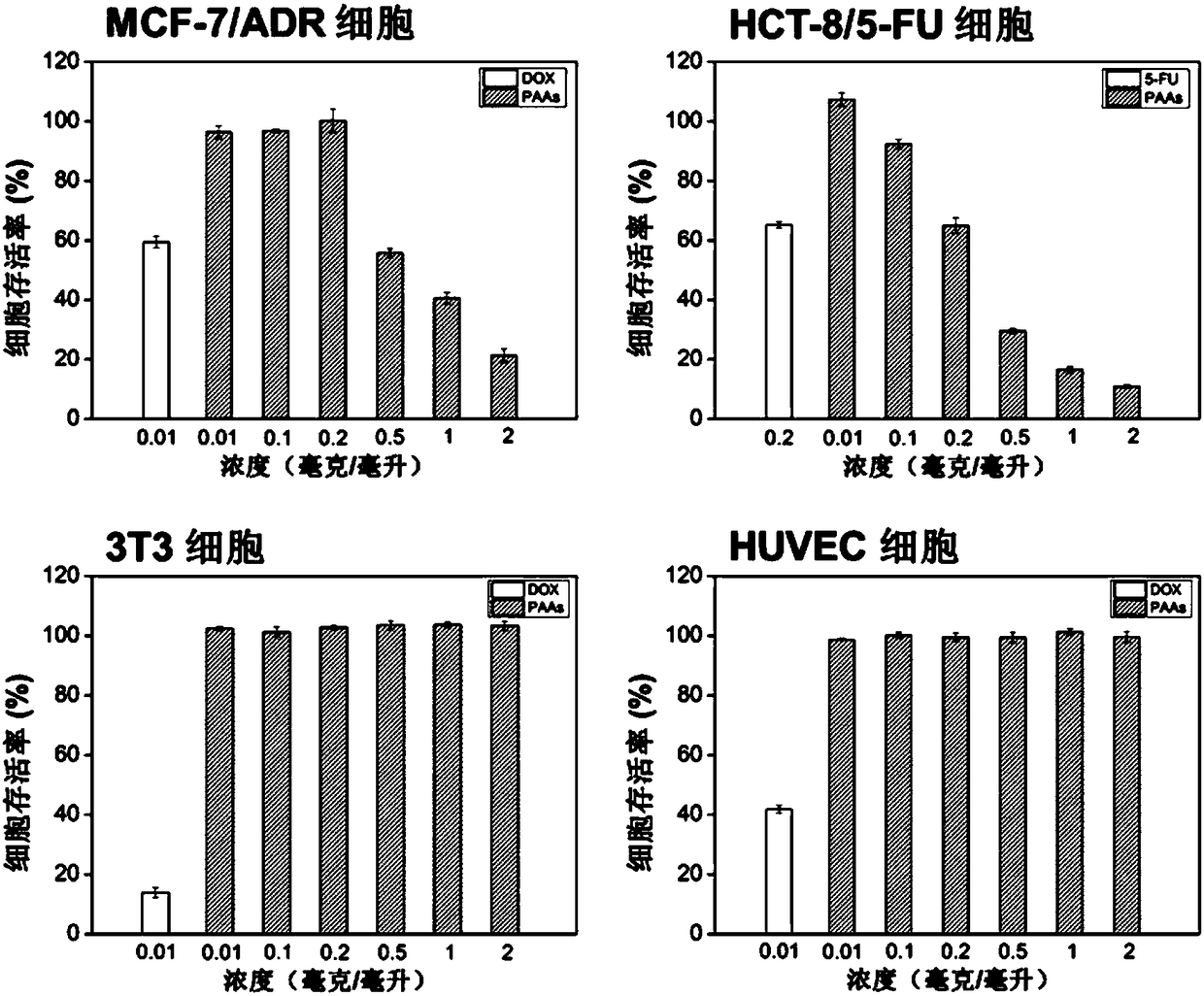
[0041] Dissolve anhydrous calcium chloride in methanol and pure water respectively, add methanol solution and aqueous solution of anhydrous calcium chloride to N,N'-bis(acryloyl)cystamine (CBA) successively, and then add N-ammonia Ethylpiperazine, condensed and refluxed at 40°C for 6 hours, magnetically stirred during the reaction, the rotation speed was 300rpm, and finally N-aminoethylpiperazine was added to block for 6 hours, the pH value was adjusted to 4, and a dialysis bag with a molecular weight cut-off of 500 was used Dialyzed for 3 days and freeze-dried to obtain N-aminoethylpiperazine-terminated hyperbranched polyamidoamines (PAAs).
[0042] The molar ratio of N,N'-bis(acryloyl)cystamine (CBA) to N-aminoethylpiperazine and anhydrous calcium chloride is 1:0.8:1.8; the N -The molar ratio of aminoethylpiperazine to N,N'-bis(acryloyl)cystamine (CBA) is 0.9:1; the amoun...
Embodiment 2
[0043] Example 2: Synthesis of N-aminoethylpiperazine-terminated hyperbranched polyamidoamines (PAAs)
[0044] Dissolve anhydrous calcium chloride in methanol and pure water respectively, add methanol solution and aqueous solution of anhydrous calcium chloride to N,N'-bis(acryloyl)cystamine (CBA) successively, and then add lysine , condensed and refluxed at 50°C for 12 hours, magnetically stirred during the reaction, and the rotation speed was 600rpm, and finally N-aminoethylpiperazine was added to block for 8 hours, the pH value was adjusted to 5, and a dialysis bag with a molecular weight cut-off of 500 was used for dialysis for 3 days. Freeze-dry to obtain N-aminoethylpiperazine-terminated hyperbranched polyamidoamines (PAAs).
[0045] The molar ratio of N,N'-bis(acryloyl)cystamine (CBA) to lysine and anhydrous calcium chloride is 1:1:2.2; the N-aminoethyl group added during capping The molar ratio of piperazine to N,N'-bis(acryloyl)cystamine (CBA) is 1.1:1; the amount of ...
Embodiment 3
[0046] Example 3: Synthesis of N-aminoethylpiperazine-terminated hyperbranched polyamidoamines (PAAs)
[0047] Dissolve anhydrous calcium chloride in methanol and pure water respectively, add methanol solution and aqueous solution of anhydrous calcium chloride to N,N'-bis(acryloyl)cystamine (CBA) in turn, and then add PEI-600 , condensed and refluxed at 50°C for 24 hours, magnetically stirred at 600 rpm during the reaction, and finally N-aminoethylpiperazine was added to block for 8 hours, and the pH value was adjusted to 6. Dialysis was performed for 3 days using a dialysis bag with a molecular weight cut-off of 1000, and then frozen After drying, N-aminoethylpiperazine-terminated hyperbranched polyamidoamines (PAAs) are obtained.
[0048] The molar ratio of N,N'-bis(acryloyl)cystamine (CBA) to PEI-600 and anhydrous calcium chloride is 1:1:2.2; the N-aminoethyl group added during capping The molar ratio of piperazine to N,N'-bis(acryloyl)cystamine (CBA) is 1.1:1; the amount of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com