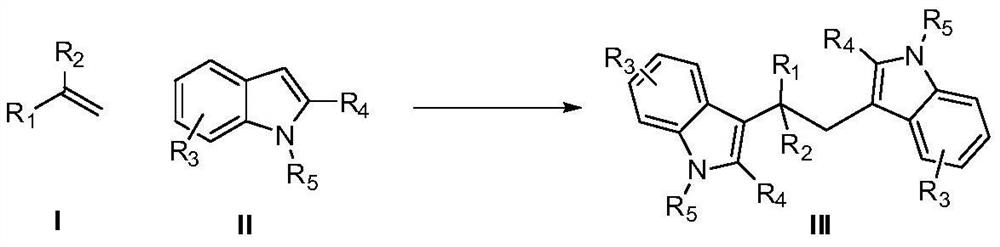
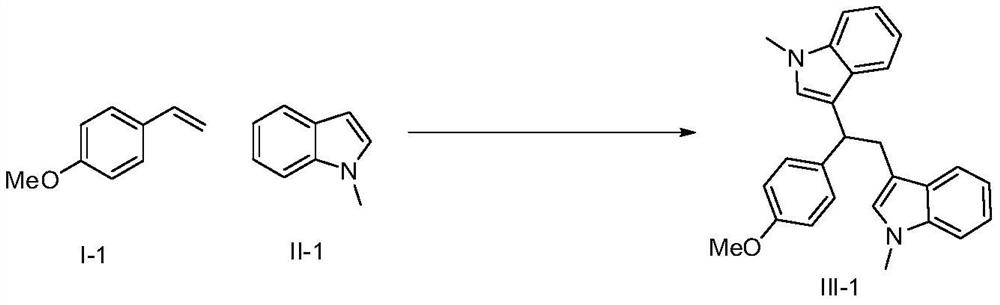
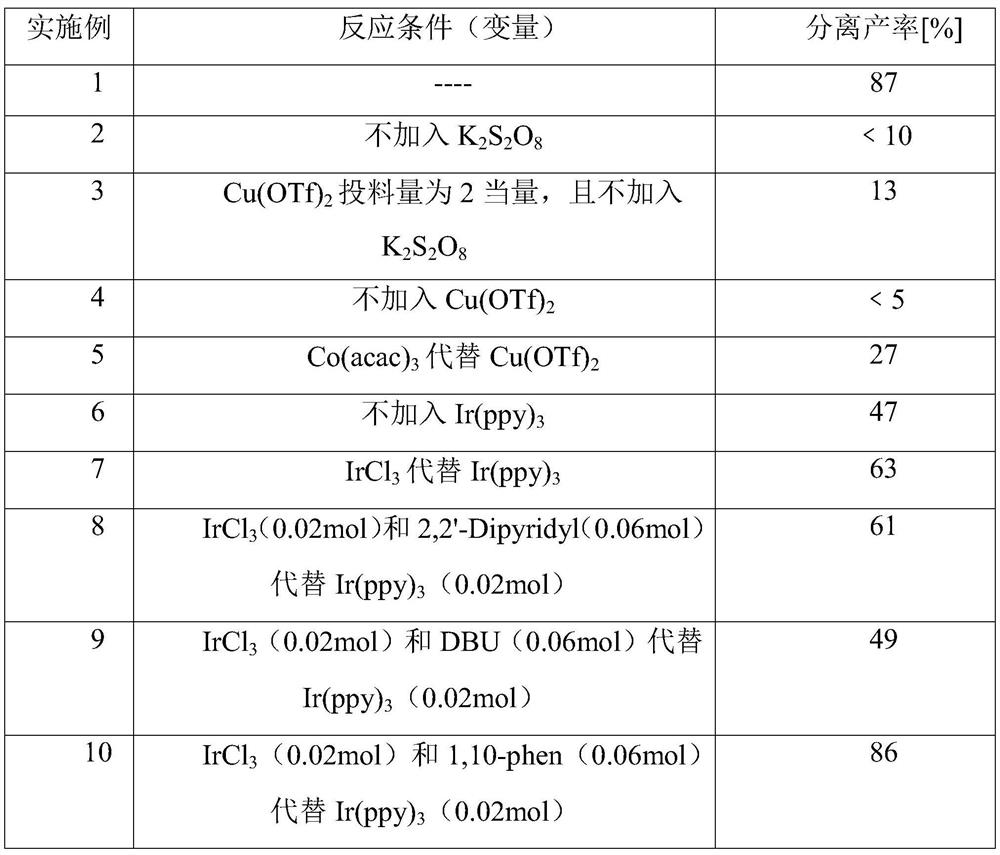
A kind of synthetic method of 3,3'-diindoleethane compounds
A technology of indoleethane and compounds, which is applied in the field of synthesis of 3,3'-diindoleethane compounds, can solve the problems of high production cost and narrow adaptability of reaction substrates, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0052]
[0053] Add p-methoxystyrene (0.2mmol) shown in formula I-1, N-ethylindole (1mmol, 5 equivalents) shown in formula II-2, Cu(OTf) in Schlenk tube lock reactor 2 (0.02mmol, 10mol%), Ir(ppy) 3 (0.02mmol, 10mol%), K 2 S 2 o 8 (0.4mmol, 2 equivalents), and MeCN (2mL), then under the protection of argon, the reaction was stirred at room temperature for 12 hours, after the completion of the reaction monitored by TLC or GC-MS, the solvent was distilled off under reduced pressure, and the residue was subjected to Column chromatography separation (eluent: n-hexane / ethyl acetate) gave the target product of formula III-2. Yield 55%. 1 H NMR (400MHz, CDCl 3 )δ:7.65-7.55(m,1H),7.46-7.37(m,1H),7.35-7.22(m,2H),7.17-7.07(m,5H),6.98(s,2H),6.81-6.68( m,2H),6.46-6.45(m,1H),4.60-4.49(m,1H),4.18-4.07(m,2H),4.01-3.94(m,2H),3.73(s,3H),3.67- 3.64(m,1H),3.37-3.32(m,1H),1.41(t,J=7.2Hz,3H),1.30-1.22(m,3H). 13 C NMR (101MHz, CDCl 3)δ: 157.7, 137.7, 136.2, 135.6, 129.0, 128.3, 127.6, 12...
Embodiment 12
[0055]
[0056] Add p-methoxystyrene (0.2mmol) shown in formula I-1, 5-cyano-N-methylindole (1mmol, 5 equivalents) shown in formula II-3 in the Schlenk lock reactor , Cu(OTf) 2 (0.02mmol, 10mol%), Ir(ppy) 3 (0.02mmol, 10mol%), K 2 S 2 o 8 (0.4mmol, 2 equivalents), and MeCN (2mL), then under the protection of argon, the reaction was stirred at room temperature for 12 hours, after the completion of the reaction monitored by TLC or GC-MS, the solvent was distilled off under reduced pressure, and the residue was subjected to Column chromatography separation (eluent: n-hexane / ethyl acetate) gave the target product of formula III-3. Yield 52%. 1 H NMR (400MHz, CDCl 3 )δ:7.56(s,1H),7.52(s,1H),7.37-7.26(m,4H),7.12(d,J=7.2Hz,2H),7.05(s,1H),6.80(d,J =7.6Hz,2H),6.69(s,1H),4.41(t,J=7.2Hz,1H),3.79(s,6H),3.70(s,3H),3.56-3.50(m,1H),3.39 -3.34(m,1H).; 3 C NMR (101MHz, CDCl 3 )δ: 158.3, 138.6, 138.1, 136.2, 129.2, 128.7, 128.1, 127.9, 127.2, 125.3, 124.5, 124.3, 120.9, 120.2, 114....
Embodiment 13
[0058]
[0059] Add p-methoxystyrene (0.2mmol) shown in formula I-1, 7-methoxycarbonyl-N-methylindole (1mmol, 5 equivalents) shown in formula II-4 in the Schlenk lock reactor ), Cu(OTf) 2 (0.02mmol, 10mol%), Ir(ppy) 3 (0.02mmol, 10mol%), K 2 S 2 o 8 (0.4mmol, 2 equivalents), and MeCN (2mL), then under the protection of argon, the reaction was stirred at room temperature for 12 hours, after the completion of the reaction monitored by TLC or GC-MS, the solvent was distilled off under reduced pressure, and the residue was subjected to Column chromatography separation (eluent: n-hexane / ethyl acetate) gave the target product of formula III-4. Yield 64%. 1 H NMR (400MHz, CDCl 3 )δ: 7.68(m, 3H), 7.51(d, J=7.6Hz, 2H), 7.12-7.04(m, 3H), 6.98-6.92(m, 2H), 6.75(d, J=8Hz, 2H) ,6.47(s,1H),4.51(t,J=7.2Hz,1H),3.93(s,6H),3.81(s,3H),3.73(s,3H),3.68(s,3H),3.60- 3.55(m,1H),3.35-3.29(m,1H) 13 C NMR (101MHz, CDCl 3 )δ: 168.1, 168.1, 157.8, 137.0, 134.3, 133.8, 132.4, 130.8, 130.8, 130...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com