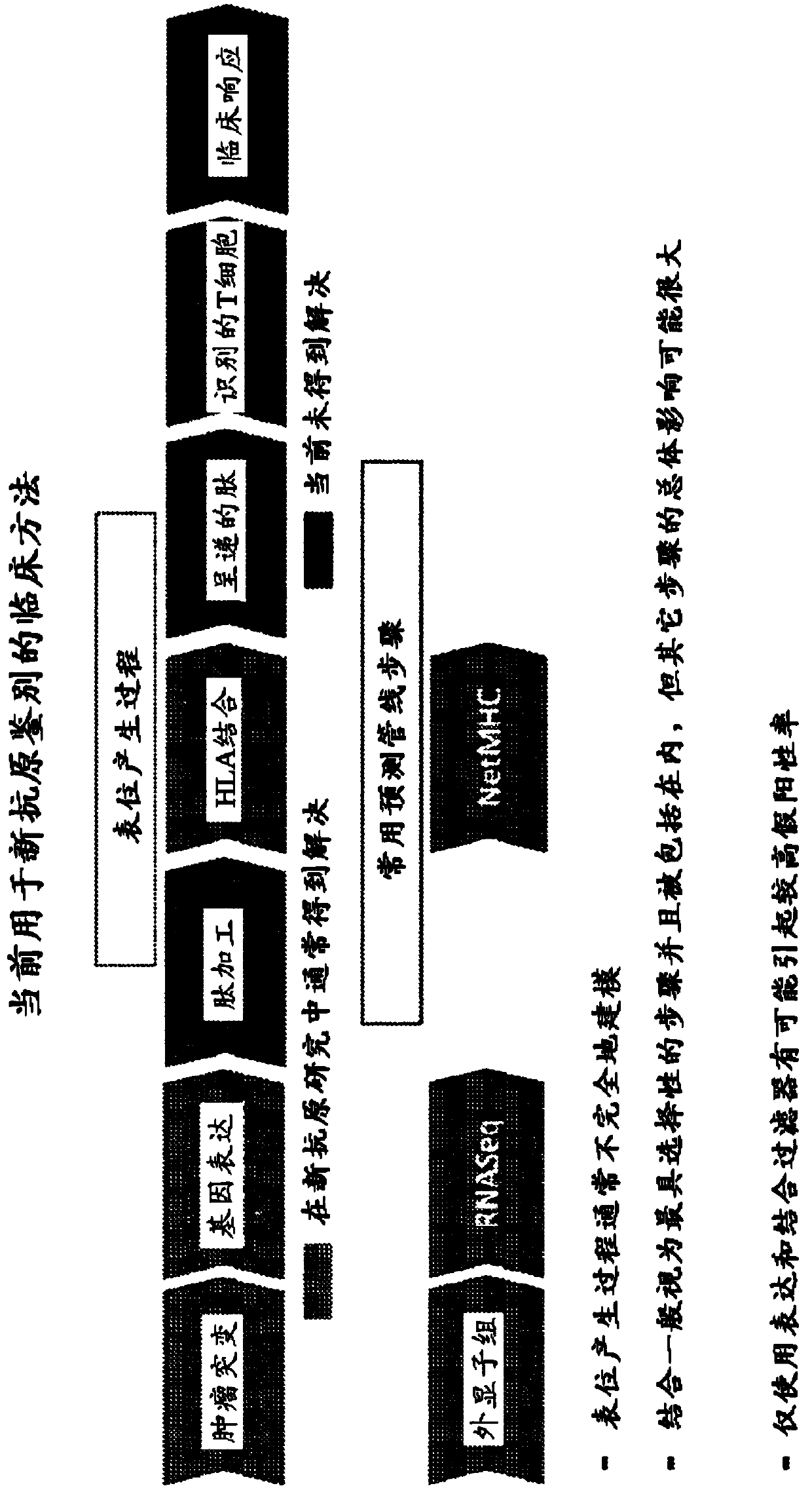
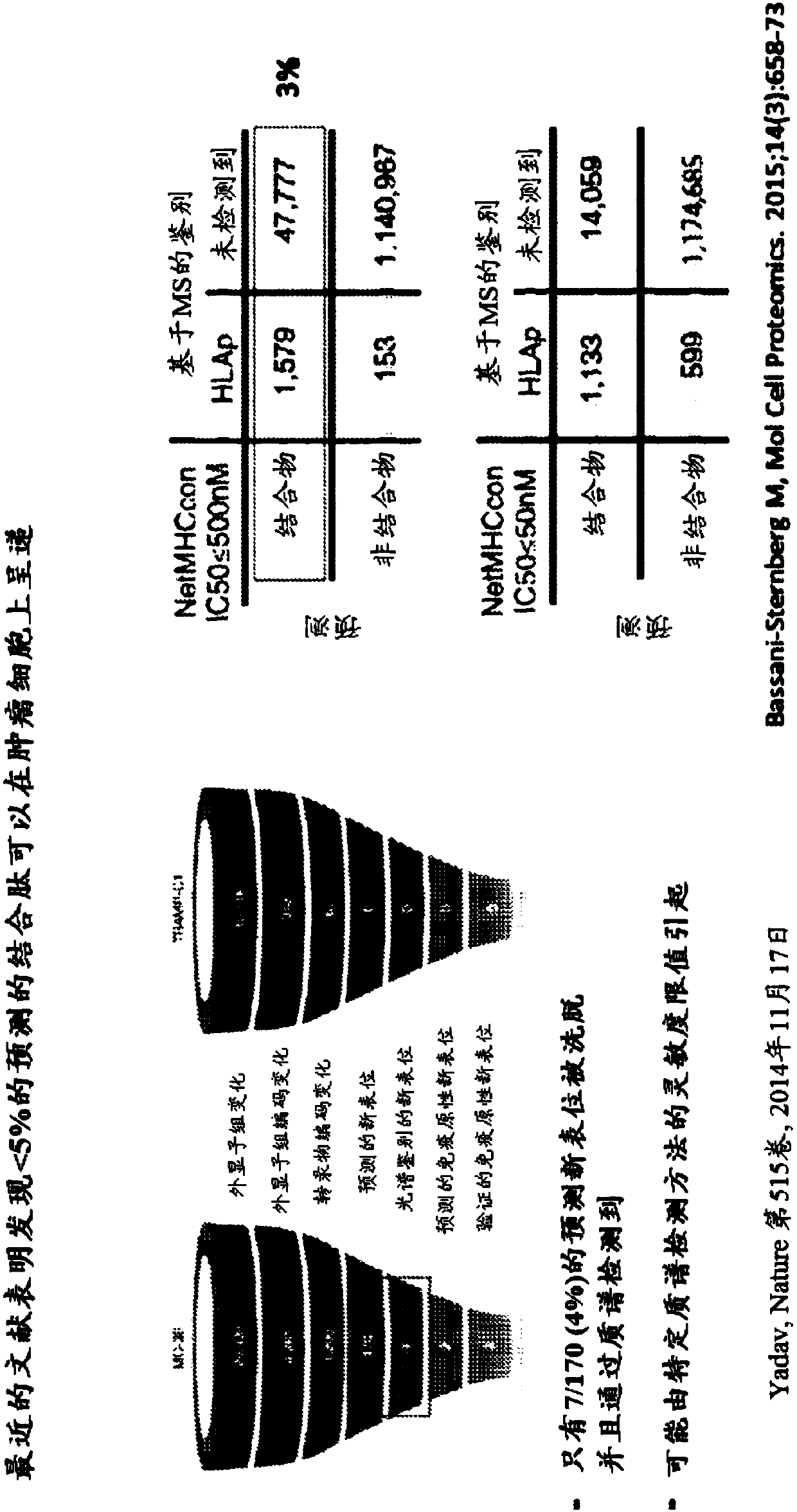
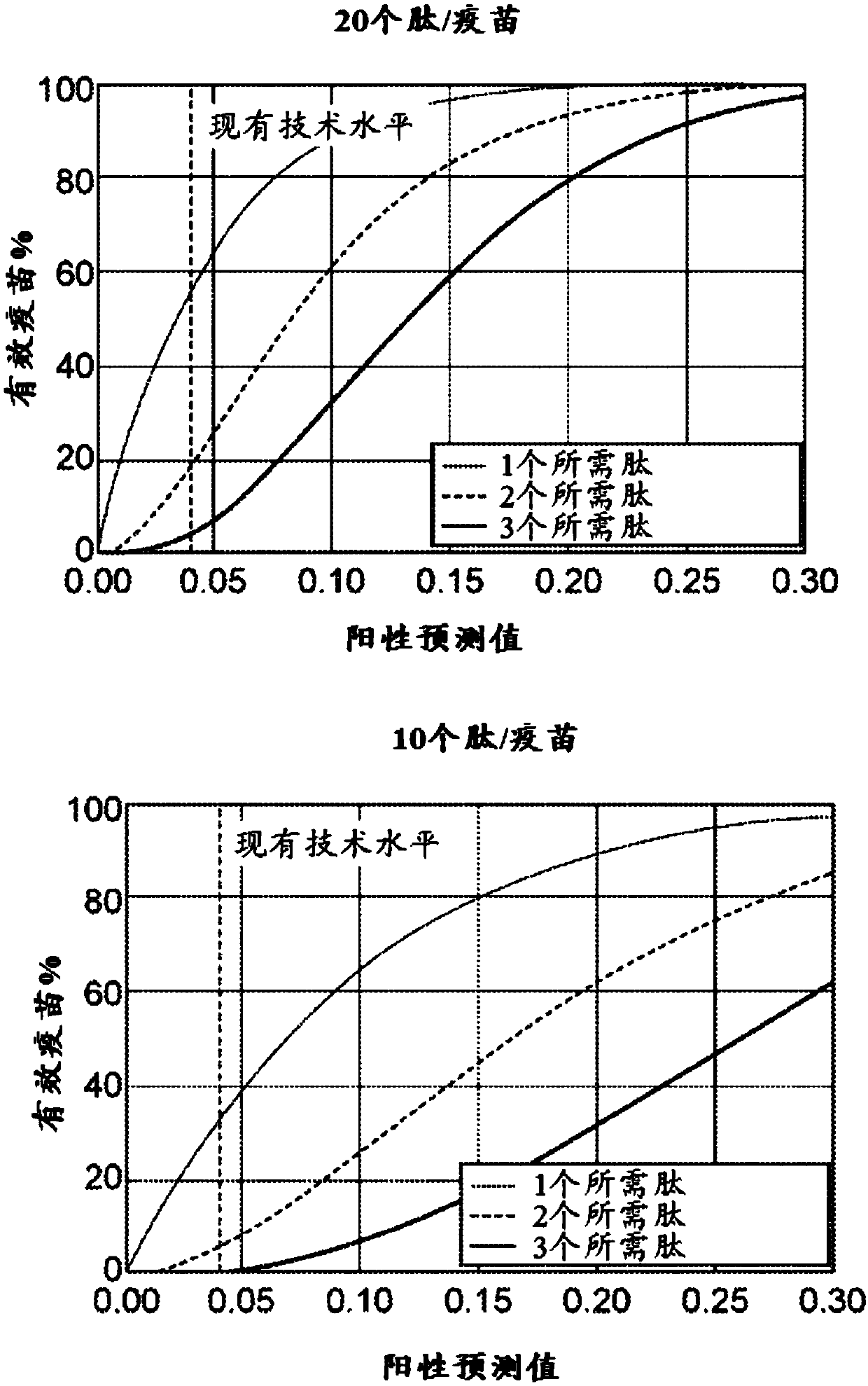
Neoantigen identification, manufacture, and use
A technology of antigens and alleles, which is applied in the direction of cancer antigen components, vertebrate antigen components, and medical preparations containing active ingredients, etc. It can solve the problems of missing candidate neoantigens and inefficient use of autoimmunity in vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0485] VIII.C.1. Example 1: Maximum of Independent Allele Models
[0486] In one embodiment, the training module 316 causes peptide p associated with a set of multiple MHC alleles H k The estimated probability of presentation u k With the probability of presentation u of each MHC allele h in set H determined based on cells expressing the monoallele k h∈H The variation of is modeled as described above in connection with equations (2)-(11). Specifically, the presentation probability u k can be u k h∈H any function of . In one embodiment, as shown in equation (12), this function is a maximum function and renders the likelihood u k can be determined as the maximum probability of presentation for each MHC allele h in set H.
[0487]
[0488] VIII.C.2. Example 2.1: The Funciton-of-Sums Model
[0489] In one embodiment, the training module 316 makes peptide p by k The estimated probability of presentation u k Modeling:
[0490]
[0491] where element a h k For...
Embodiment 22
[0501] VIII.C.3. Example 2.2: Functional Model Using the Sum of Allelic Non-Interacting Variables
[0502] In one embodiment, the training module 316 incorporates allelic non-interaction variables and makes peptide p by k The estimated probability of presentation u k Modeling:
[0503]
[0504] where w k Indicates the encoded related peptide p k The allelic non-interaction variable for . Specifically, the parameter set θ for each MHC allele h h and the set of parameters about the allelic non-interacting variables θ w The value of θ can be obtained by making about θ h and θ w is determined by minimizing a loss function of , where i is each instance in the subset S of training data 170 produced by cells expressing a single MHC allele and / or by cells expressing multiple MHC alleles. Correlation function g w The correlation function g introduced in Section VIII.B.3 above can be expressed as w any of the forms.
[0505] Therefore, according to equation (14), the fun...
Embodiment 31
[0516] VIII.C.4. Example 3.1: Models Using Implicit Independent Allele Likelihoods
[0517] In another embodiment, the training module 316 makes the peptide p by k The estimated probability of presentation u k Modeling:
[0518]
[0519] where element a h k For the peptide sequence p k Associated multiple MHC alleles h ∈ H is 1, u' k h is the implicit independent allelic presentation likelihood of the MHC allele h, and the vector v is the element v h corresponds to a h k u' k h , s(·) is a function that maps element v, and r(·) is a clipping function that clips the input values into a given range. As described in more detail below, s(·) may be a sum function or a second order function, but it is understood that in other embodiments, s(·) may be any function, such as a maximum function. The value of the parameter set θ with respect to the likelihood of implicitly independent alleles can be determined by minimizing a loss function with respect to θ, where i is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com