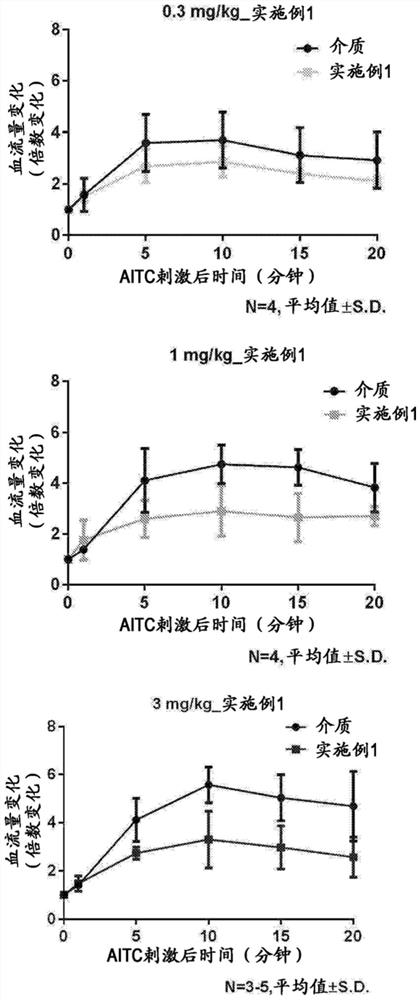
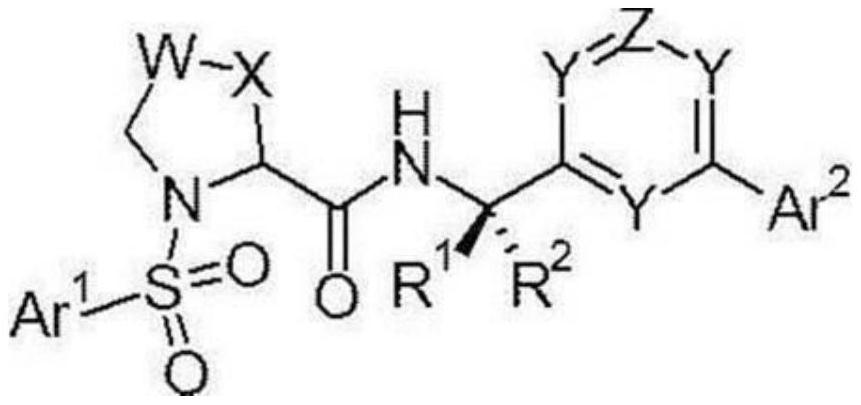
Heterocyclic sulfonamide derivatives and medicines containing them
A drug and pharmaceutical technology applied in the field of novel heterocyclic sulfonamide compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0721] Hereinafter, the present invention will be described in detail using reference examples, examples, and test examples, but the present invention is not limited to these. In addition, unless otherwise specified, the devices, reagents, and the like used in this example can be easily prepared according to methods generally practiced in the art, or can be purchased commercially. In addition, % of the title compound represents a yield.
[0722] The structural formulas and physical properties of the reference example compounds are shown in Table 2.
[0723] [table 2-1]
[0724]
[0725] [Table 2-2]
[0726]
[0727] [Table 2-3]
[0728]
[0729] [Table 2-4]
[0730]
[0731] [Table 2-5]
[0732]
reference example A-1
[0733] Reference Example A-1: Synthesis of 5-fluorobenzofuran-2-ylsulfonyl chloride (A-1)
[0734] (Step 1) Synthesis of 2-(2,2-dibromovinyl)-4-fluorophenol
[0735] A solution of carbon tetrabromide (1.70kg, 5.14mol) in dichloromethane (80mL) was cooled to 0°C, triphenylphosphine (2.07kg, 7.91mol) was added, and stirred for 30 minutes. Triethylamine (1.30 kg, 12.8 mol) was added to the reaction mixture, and then 5-fluoro-2-hydroxybenzaldehyde (300 g, 2.14 mol) was added slowly while keeping the reaction temperature below 5°C. The reaction mixture was stirred at 30°C for 2 hours, then concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (hexane / ethyl acetate) to obtain the title compound (300 g, 1.01 mol, 47%).
[0736] 1 H NMR (400MHz, CDCl 3 )δ7.54 (s, 1H), 7.35-7.32 (m, 1H), 6.95-6.90 (m, 1H), 6.79-6.75 (m, 1H), 5.41 (s, 1H).
[0737] (Step 2) Synthesis of 2-bromo-5-fluorobenzofuran
[0738] Tetrahydrofuran...
reference example A-2
[0744] Reference Example A-2: Synthesis of 4-fluorobenzofuran-2-ylsulfonyl chloride (A-2)
[0745] The title compound was obtained by the same procedure as in Reference Example A-1, using 6-fluoro-2-hydroxybenzaldehyde instead of 5-fluoro-2-hydroxybenzaldehyde.
[0746] MS(ESI)m / z 235(M+H) +
[0747] 1 H NMR (400MHz, CDCl 3 ) δ 7.72 (s, 1H), 7.60-7.55 (m, 1H), 7.47 (d, J=8.8Hz, 1H), 7.12 (t, J=8.8Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com