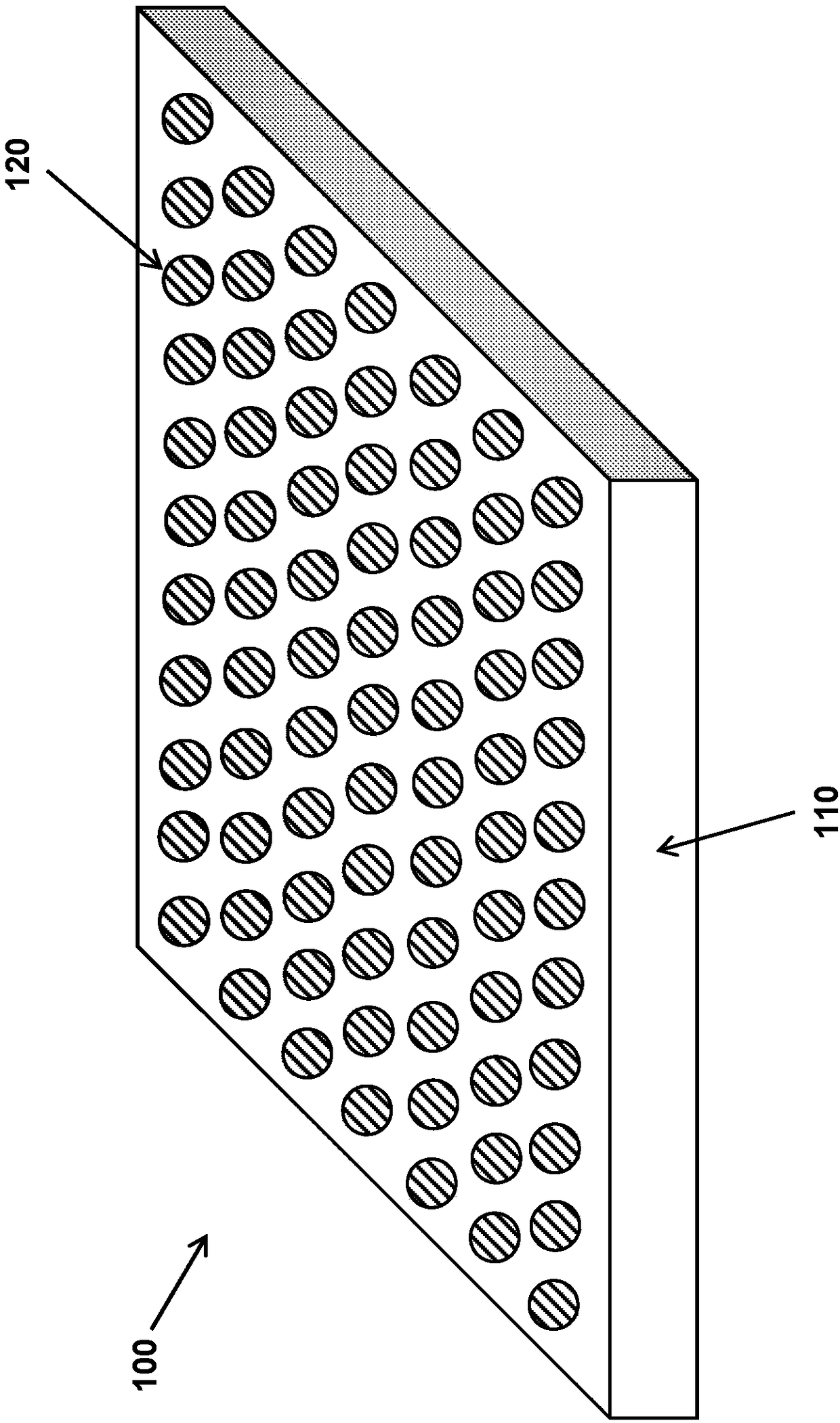
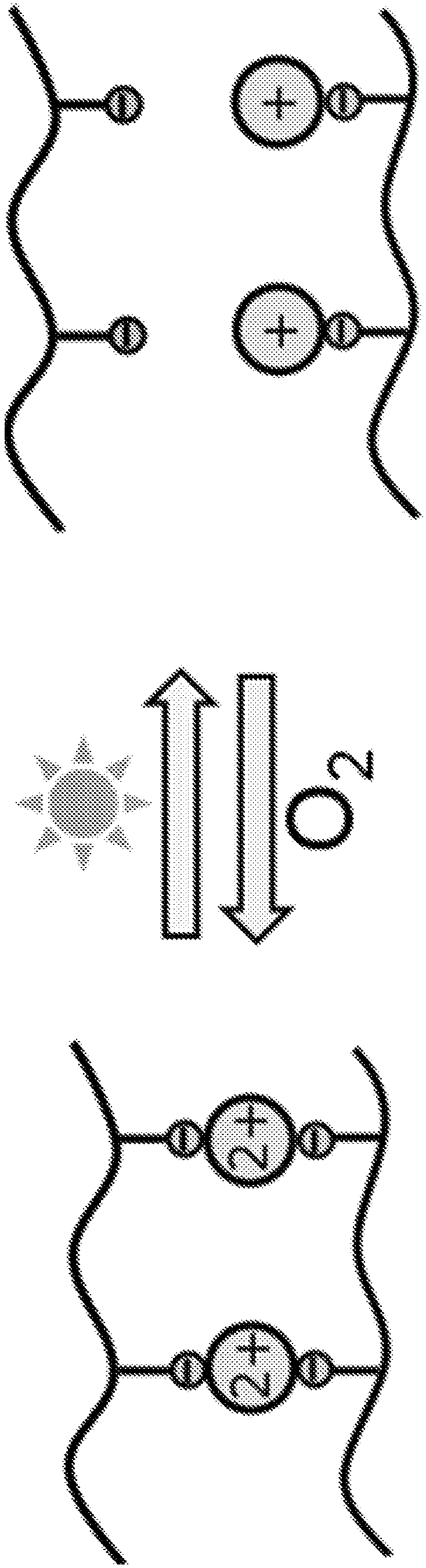
Reversible, chemically or environmentally responsive polymers, and coatings containing such polymers
A technology of polymers, chemistry, applied in the field of polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
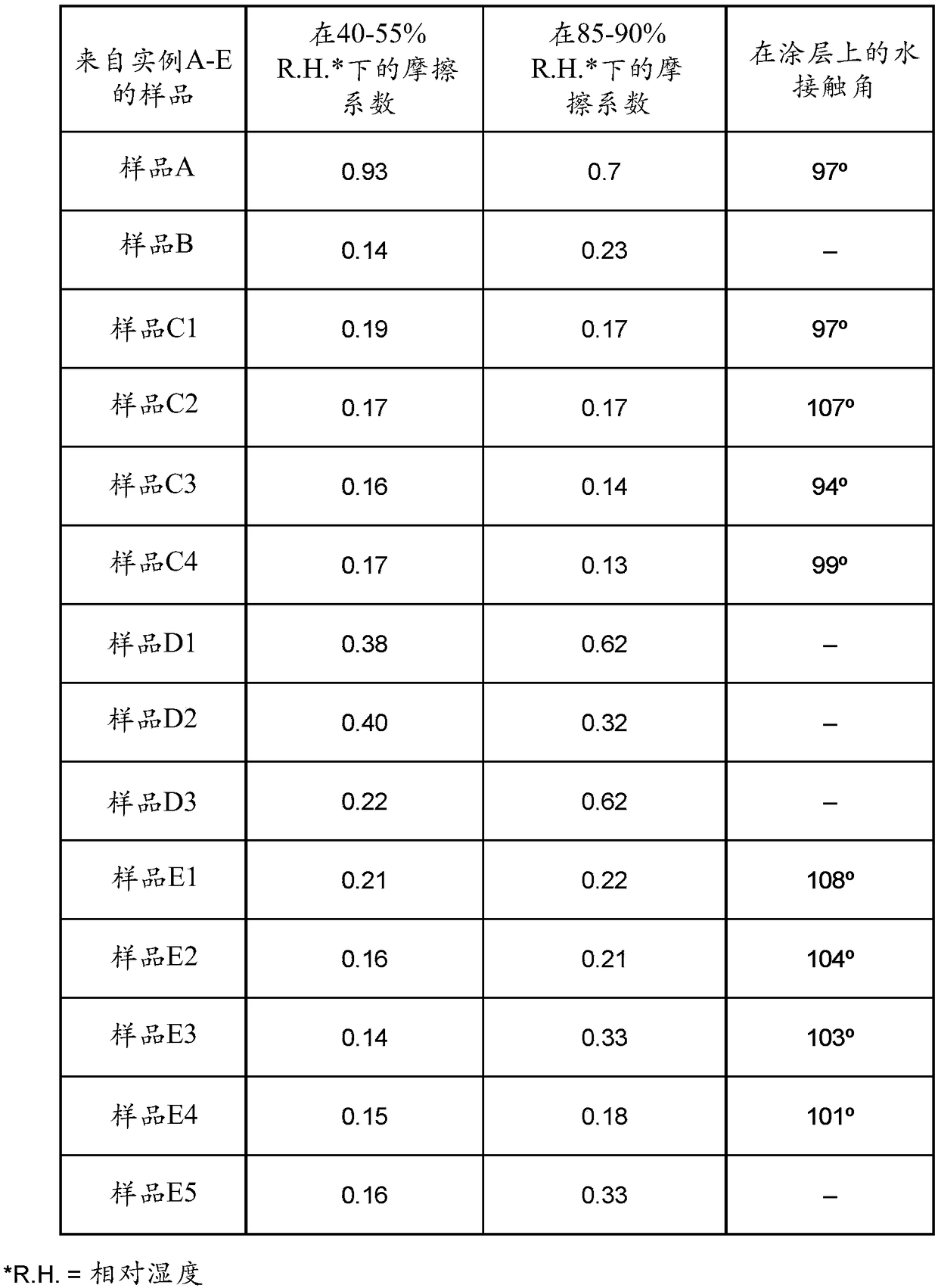
[0254] Material. Has a molecular weight of 3,400 g / mol (M n ) of poly(ethylene glycol) (PEG), 4,4'-methylenebis(cyclohexylisocyanate) (HMDI), 1,4-butanediol (BD), dibutyltin dilaurate ( DBTDL), and 2,2-bis(hydroxymethyl)propionic acid were purchased from Aldrich. Fluorolink material was purchased from Solvay Specialty Polymers. All chemicals were used without further purification.
example A
[0255] Example A: Fluoropolymer Control.
[0256] A vial was charged with Fluorolink D4000 perfluoropolyether (4 g), followed by HMDI (0.786 g) and dibutyltin dilaurate (0.02%). A small PTFE-coated stir bar was introduced and the vial was placed in a 100°C oil bath for stirring. After achieving a temperature of 100°C the reaction was vortexed vigorously, then stirring was continued for 1 hour. After this step, the resin was poured into a 3"x3"PTFE mold to flash off the solvent and the film was allowed to cure overnight at room temperature.
example B
[0257] Example B: Thermoplastic polymer without ionic species.
[0258] A hydroxyl-terminated poly(perfluoropolyether) (9.00 g, 3.73 mmol, Fluorolink 5147x) was placed in a 3-neck round bottom flask containing an argon inlet and equipped with an overhead stirrer (Teflon shaft and blades ). While stirring, 4,4'-methylenebis(cyclohexylisocyanate) (4.89 g, 18.66 mmol) was added to the solution and the flask was placed in an oil bath at 100°C. Dibutyltin dilaurate (0.02% by weight) was then added to the solution using a micropipette and polymerization was allowed to proceed.
[0259] After 1 h, the prepolymer was then allowed to cool to room temperature. The prepolymer was diluted with tetrahydrofuran (15 mL) and placed in a centrifugal mixer (FlackTek DAC 600).
[0260] In a separate vial, the chain extender 1,4-butanediol (1.35 g, 14.98 mmol) was weighed out and diluted with tetrahydrofuran (0.5 mL). The two solutions were combined in a centrifugal mixer and mixed for 15 sec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com