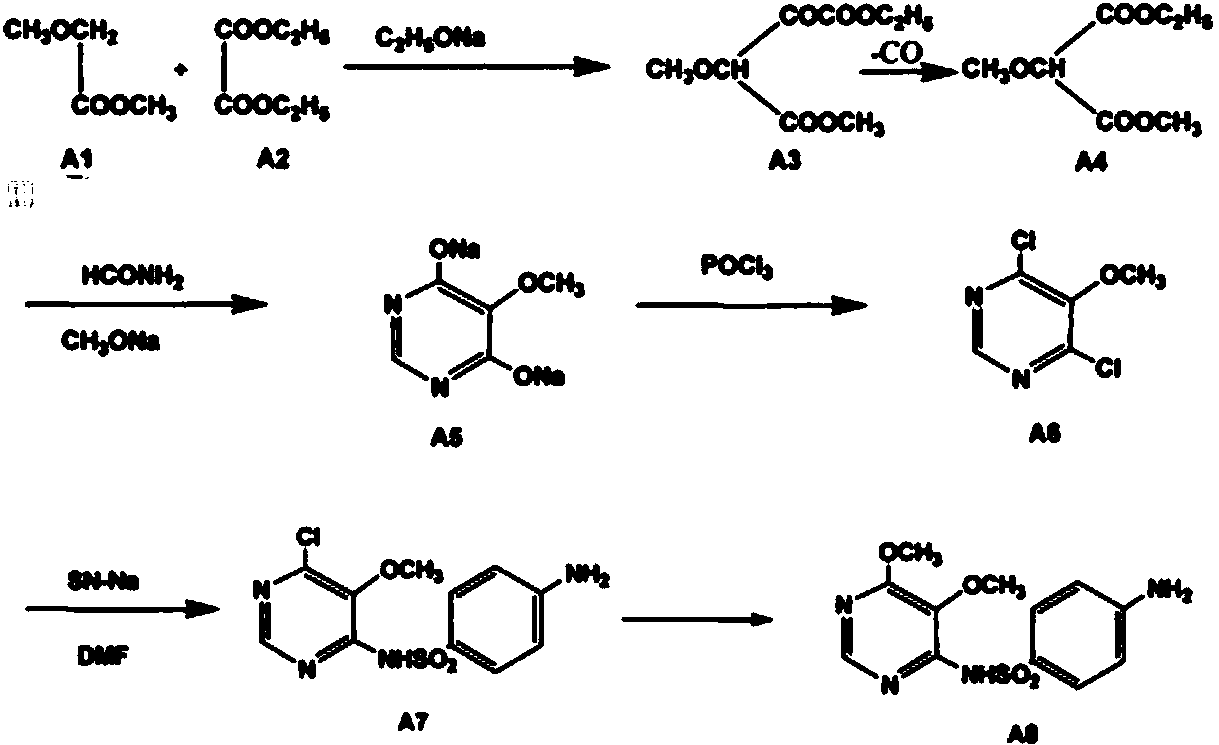
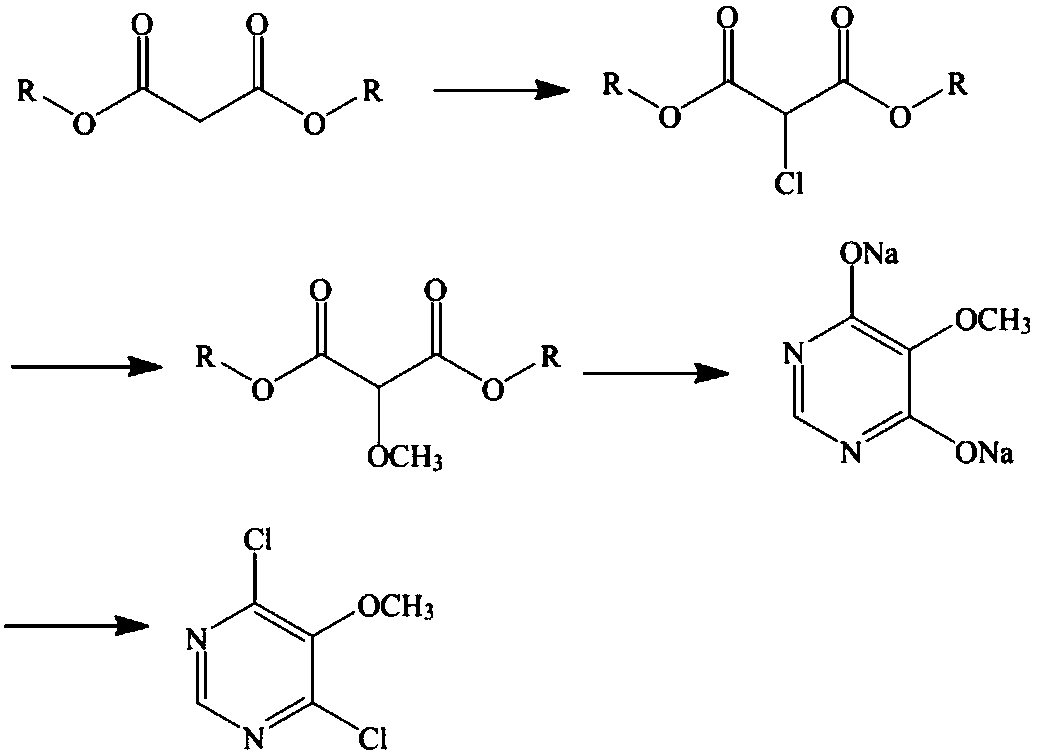
Preparation method of sulfadoxine midbody 4,6-dichloro-5-methoxypyrimidine
A methoxy pyrimidine and methoxy pyrimidine technology is applied in the field of preparation of 4,6-dichloro-5-methoxy pyrimidine as a periodic sulfonamide intermediate, which can solve the problems of low production efficiency, unfavorable continuous production, Problems such as many purification steps, to achieve the effect of improving yield, improving industrial production efficiency, and reducing purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A preparation method of sulfonamide intermediate 4,6-dichloro-5-methoxypyrimidine, the steps are as follows:
[0047] 1) Preparation of 2-chloro-dimethyl malonate
[0048] Add 800L of dichloromethane into the No. 1 reaction kettle with a diaphragm pump, start stirring, add 400kg of dimethyl malonate, continue stirring and control the temperature at 8°C, open the tail gas absorption device, and start to introduce chlorine gas, and the chlorine gas and propane The molar ratio of dimethyl malonate is 1:0.812, and the passage of dimethyl malonate is completed in 3 hours; after the addition of chlorine gas, the temperature is raised to 25° C., and the reaction is carried out for 4 hours; sampling, detection by high-efficiency gas chromatography, the remaining dimethyl malonate does not exceed 1%, depending on For the completion of the chlorination reaction; concentrate under reduced pressure to remove dichloromethane, and take away excess chlorine gas; directly add 200L of m...
Embodiment 2
[0056] A kind of preparation method of sulfonamide intermediate 4,6-dichloro-5-methoxypyrimidine,
[0057] It differs from Example 1 in that:
[0058] 1) Preparation of 2-chloro-di-tert-butyl malonate
[0059] Add 800 L of dichloroethane into the No. 1 reactor with a diaphragm pump, start stirring, add 654.8 kg of di-tert-butyl malonate, continue stirring and control the temperature at 8°C, start the tail gas absorption device, and start to introduce chlorine gas, and The molar ratio of chlorine gas to di-tert-butyl malonate is 1:0.812, and the passage is completed in 3 hours; after the addition of chlorine gas, the temperature is raised to 25° C., and the reaction is carried out for 4 hours; sampling is carried out and detected by high performance gas chromatography, and there is no remaining di-tert-butyl malonate If it exceeds 1%, it is considered that the chlorination reaction is complete; the dichloroethane is removed by concentration under reduced pressure, and the exce...
Embodiment 3
[0065] A kind of preparation method of sulfonamide intermediate 4,6-dichloro-5-methoxypyrimidine,
[0066] It differs from Example 1 in that:
[0067] 1) Preparation of 2-chloro-dimethyl malonate
[0068] Add 800L of dichloromethane into the No. 1 reaction kettle with a diaphragm pump, start stirring, add 400kg of dimethyl malonate, continue stirring and control the temperature at 8°C, open the tail gas absorption device, and start to introduce chlorine gas, and the chlorine gas and propane The molar ratio of dimethyl malonate is 1:0.812, and the passage is completed in 3 hours; after the addition of chlorine gas, start to heat up to 25° C., and react for 8 hours; sampling, high performance gas chromatography detection, dimethyl malonate remains no more than 1%, depending on In order to complete the chlorination reaction; concentrate under reduced pressure to remove dichloromethane, and take away excess chlorine gas; directly add 200L of methanol to the remaining liquid to di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com