Tartrate salt of selective cdk9 inhibitor and its crystal form
A technology of tartrate salt and crystal form, which is applied in the field of chemical pharmacy, can solve the problems of unsalted species, further evaluation of performance, and unprepared, etc., and achieve good crystallinity, easy production scale expansion, and good stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Salt-forming performance of 3-(5-fluoro-4-(4-methyl-2-(methylamino)thiazol-5-yl)pyrimidin-2-ylamino)-benzenesulfonamide (code LS007)
[0054] 1.1 High-throughput screening of salt formation
[0055] Combined with the pKa value of LS007 and the solubility in different pHs, it can be determined that acids with a pKa value of about 3 or less can be used as acids for salt-forming screening. Therefore, we selected 8 acids: hydrochloric acid, sulfuric acid, aspartic acid, maleic acid, phosphoric acid, glutamic acid, tartaric acid, fumaric acid.
[0056]The drug was dissolved and added to a 96-well plate, and the volume of the added counterion was determined according to the molar amount of the added drug and the number of counterion functional groups. Heating time and temperature can be determined according to specific conditions (generally 40 ℃, 1 hour). In order to ensure a certain pressure during the reaction in the bottle, it is necessary to ensure the absolut...
Embodiment 2
[0072] Example 2 Crystal form of 3-(5-fluoro-4-(4-methyl-2-(methylamino)thiazol-5-yl)pyrimidin-2-ylamino)-benzenesulfonamide tartrate
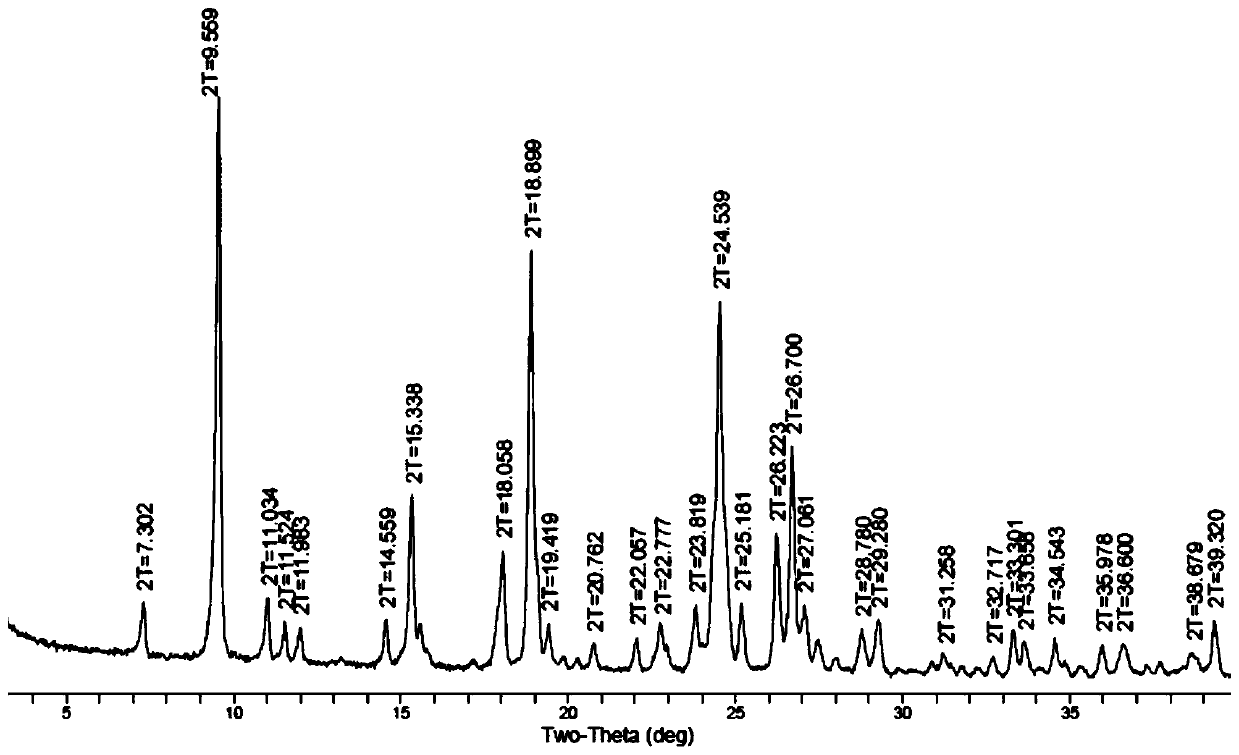
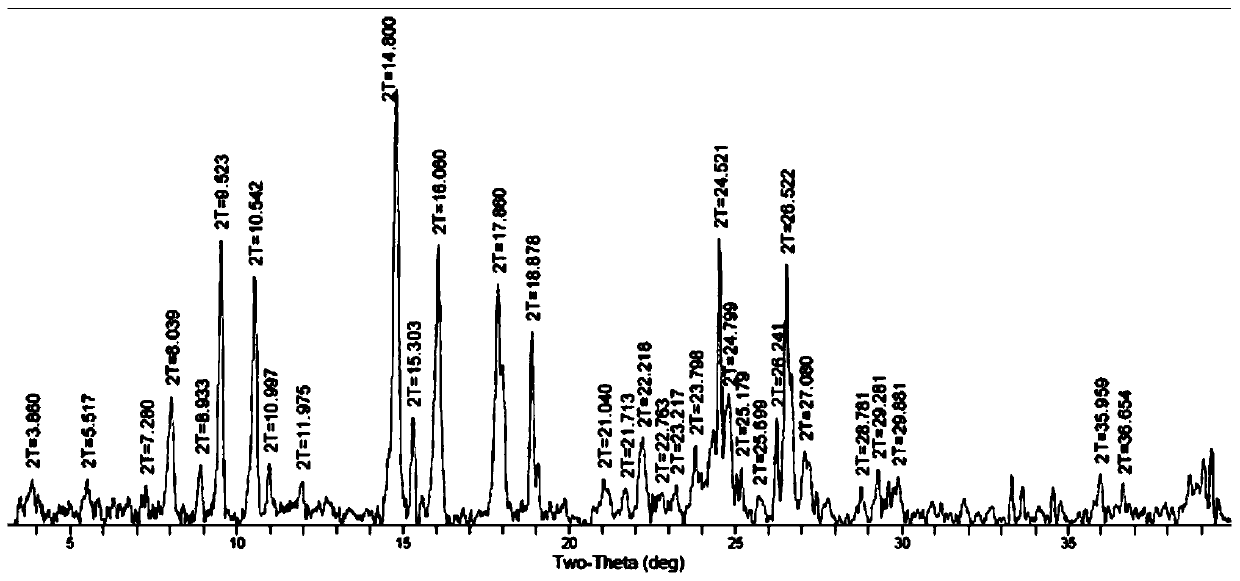
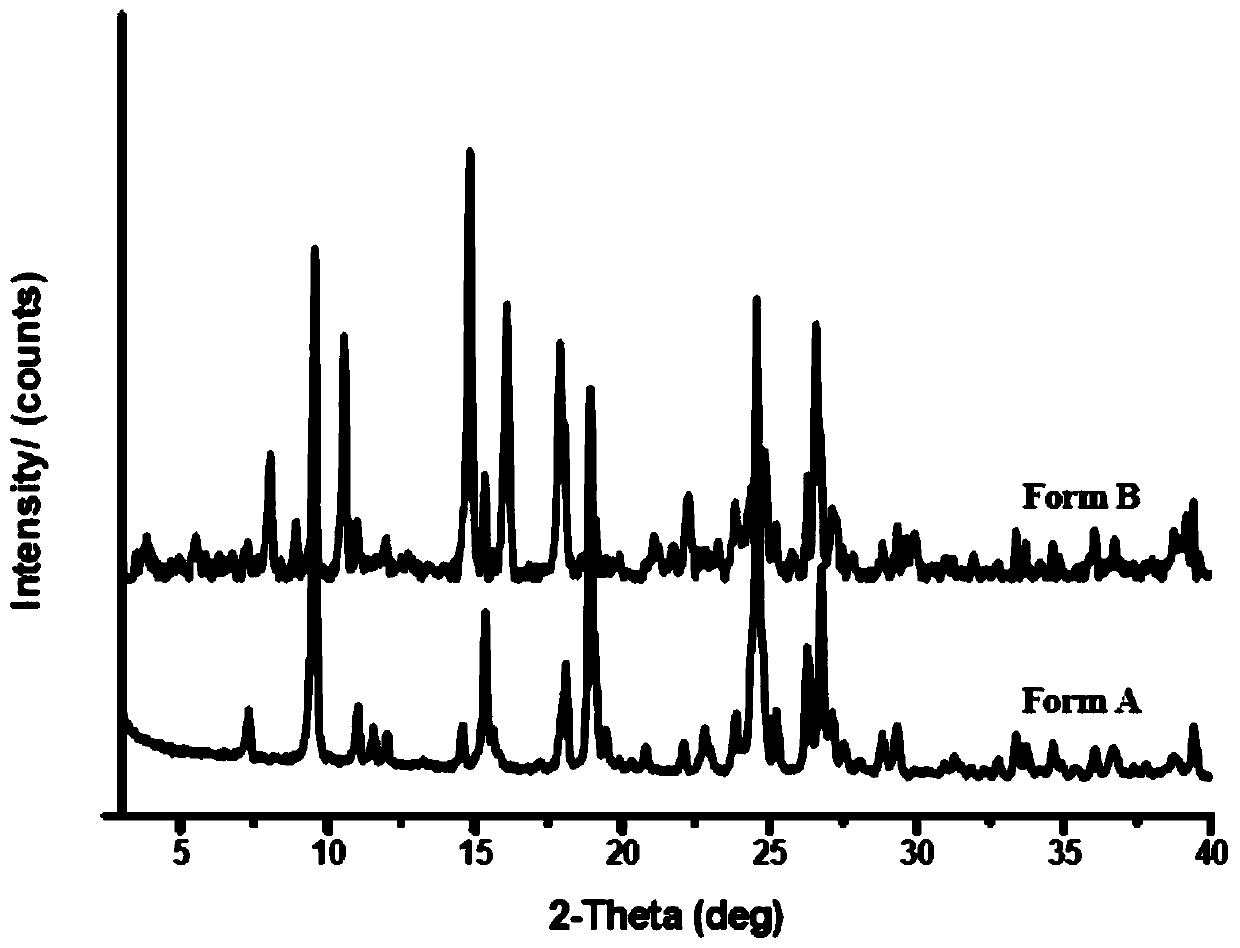
[0073] In this study, aiming at the polymorphic form of LS007 tartrate, different crystallization conditions and experimental methods were used to systematically screen the possible crystal forms of compound LS007 tartrate. Through nearly 300 crystallization experiments, it was found that LS007 tartrate can exist in two different crystal forms, which are crystal forms A and B respectively. Further characterization found that there was no significant difference in physical and chemical properties between different crystal forms. In the transformation experiment between crystal forms, it was found that Form A is a more stable crystal form, and Form B can be transformed into Form A crystal form under certain conditions.
[0074] (1) Form A
[0075] Columnar crystals, the drug melts and decomposes, with a peak decomposition temperature of 236.8°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com