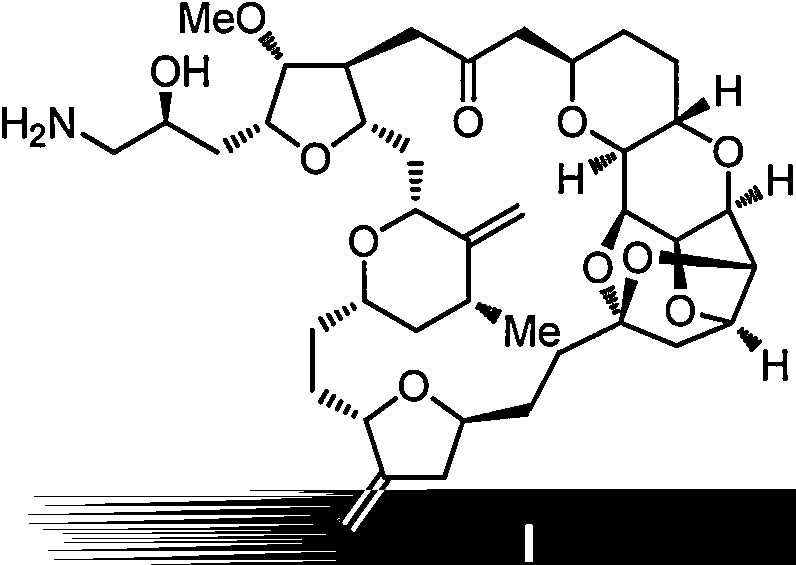
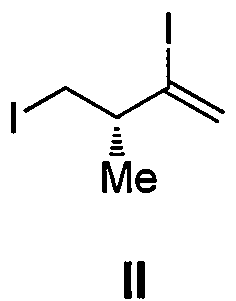
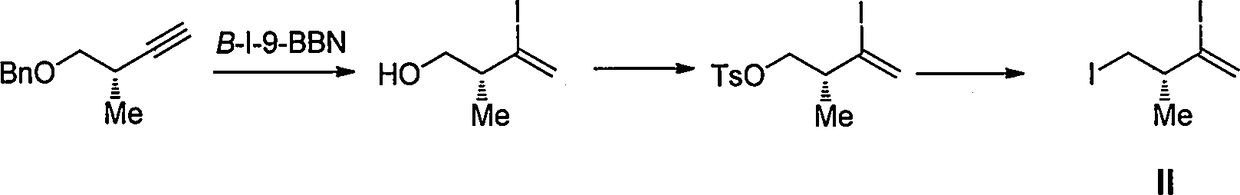
Intermediate for preparing eribulin, and preparation method thereof
A compound and a defined technology, applied in the field of intermediates for the preparation of eribulin, can solve the problems of unsuitability for large-scale production, harsh reaction conditions, and potential safety hazards, and achieve the effects of low synthesis cost, easy operation, and frequent replacement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Embodiment 1: preparation compound VIIa
[0171] Weigh VIa (10g, 36.7mmol) and MeONHMe·HCl (4.6g) into the flask, add 100mL THF to suspend, and slowly add 2.0M isopropylmagnesium chloride-tetrahydrofuran solution (30mL) to it at -30~0°C . After the reaction was stirred at -30~0°C for 30 minutes, TLC showed complete conversion of starting material. The reaction was quenched by adding saturated aqueous ammonium chloride solution, extracted with ethyl acetate, concentrated and purified to obtain compound VIIa (10.3 g).
[0172] MS(ESI)m / z: 302(M+H + )
[0173] 1 H NMR (400MHz, Chloroform-d) δ7.87-7.73 (m, 2H), 7.36 (d, J=8.2Hz, 2H), 4.22 (dd, J=9.5, 8.2Hz, 1H), 3.99 (dd, J=9.5, 6.1Hz, 1H), 3.72(s, 3H), 3.35-3.30(m, 1H), 3.17(s, 3H), 2.46(s, 3H), 1.11(d, J=7.0Hz, 3H ).
Embodiment 2
[0174] Embodiment 2: Preparation of compound VIIa
[0175] VIa (10 g, 36.7 mmol), MeONHMe·HCl (4.6 g) and potassium carbonate (3.2 g) were weighed into the flask, suspended in 100 mL of DMF, stirred at 50° C. for 6 h, and TLC showed complete conversion of the raw materials. The reaction was quenched by adding saturated aqueous ammonium chloride solution, extracted with ethyl acetate, concentrated and purified to obtain compound VIIa (9.8 g).
Embodiment 3
[0176] Embodiment 3: preparation compound VIIa
[0177] Via (10 g, 36.7 mmol), MeONHMe (6.5 g) and diisopropylethylamine (4.9 mL) were weighed into the flask, 200 mL of toluene was added, and after stirring at 80° C. for 12 h, TLC showed complete conversion of the starting material. The reaction was quenched by adding saturated aqueous ammonium chloride solution, extracted with ethyl acetate, concentrated and purified to obtain compound VIIa (9.6 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com