Immunity enhancing method for Lp-PLA2, NGAL, and Derf24
A cationic carrier and protein technology, which is used in the field of Lp-PLA2, NGAL and Derf24 to enhance immunity, and can solve the problems that cannot meet the detection requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of cationized bovine serum albumin (c510BSA for short)
[0047] Use 1,5-pentanediamine and 1,10-decanediamine to prepare cationic bovine serum albumin by modifying natural bovine serum albumin. The specific steps of modification are as follows:
[0048] (1), add 200mg of 1,5-pentanediamine (CAS: 462-94-2) and 168.6mg of 1,10-decanediamine (CAS: 646-25-3) into 20ml H 2 In O, adjust the pH to 4.75 with 6N HCl, adjust the total volume to 50ml, and equilibrate to room temperature (25°C);
[0049] (2), add 737.2mg BSA (dissolved in 5ml H 2 in O);
[0050] (3), add 220 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (hereinafter referred to as EDC, CAS: 25952-53-8) to the above solution, stir at room temperature for 1 Hour;
[0051] (4), prepare 4M pH=4.75 acetic acid buffer solution and add 3.5ml to terminate the reaction of the above solution;
[0052] (5), dialyze the above solution with 50mM pH=6MES;
[0053] (6) After measur...
Embodiment 2
[0054] Example 2: Coupling and synthesis of c510BSA-LP-PLA2 immunogen
[0055] The specific steps of coupling of cationized bovine serum albumin c510BSA and recombinant human lipoprotein-related phospholipase A2 are as follows:
[0056] (1), take 1ml of LP-PLA2 recombinant protein (R&D company product, 5mg) and dissolve it in 1ml MES (pH6.0) buffer solution, a total of three groups;
[0057] (2) Add 4.3 mg of EDC to the protein solution and react at room temperature for 5 minutes;
[0058] (3), with 0.5M Na 2 HPO 4 Adjust to pH=7.2;
[0059] (4), add 5.9 mg of N-hydroxysuccinimide (NHS) to the above solution and react at room temperature for 20 minutes;
[0060] (5), 2 mg of cationized bovine serum albumin c510BSA, nBSA (unmodified), and KLH were added to the protein solution and reacted for 1.5 hours;
[0061] (6) The protein coupling mixture was dialyzed into PBS (pH7.2), and the c510BSA-LP-PLA2 recombinant protein immunogen, nBSA-LP-PLA2 recombinant protein immunogen, ...
Embodiment 3
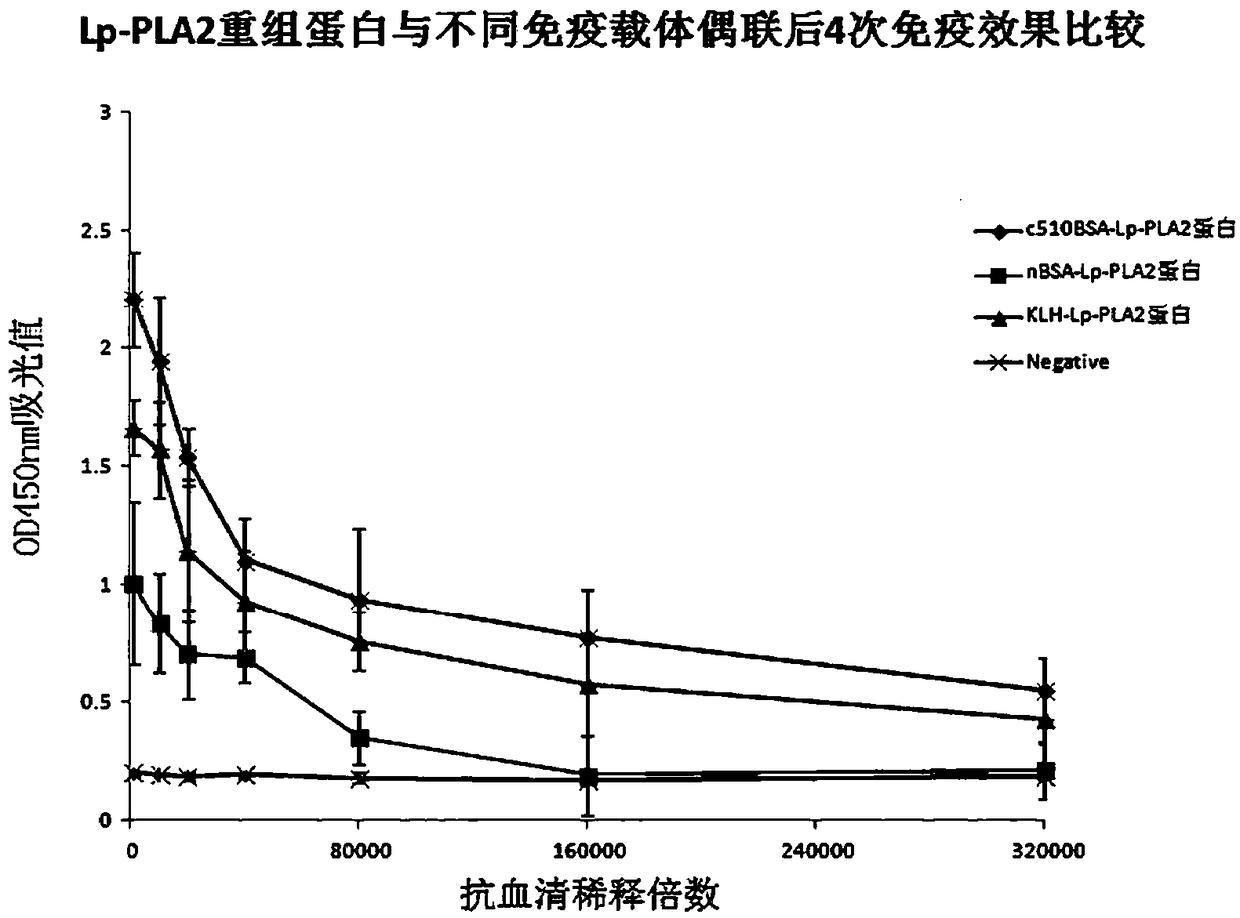
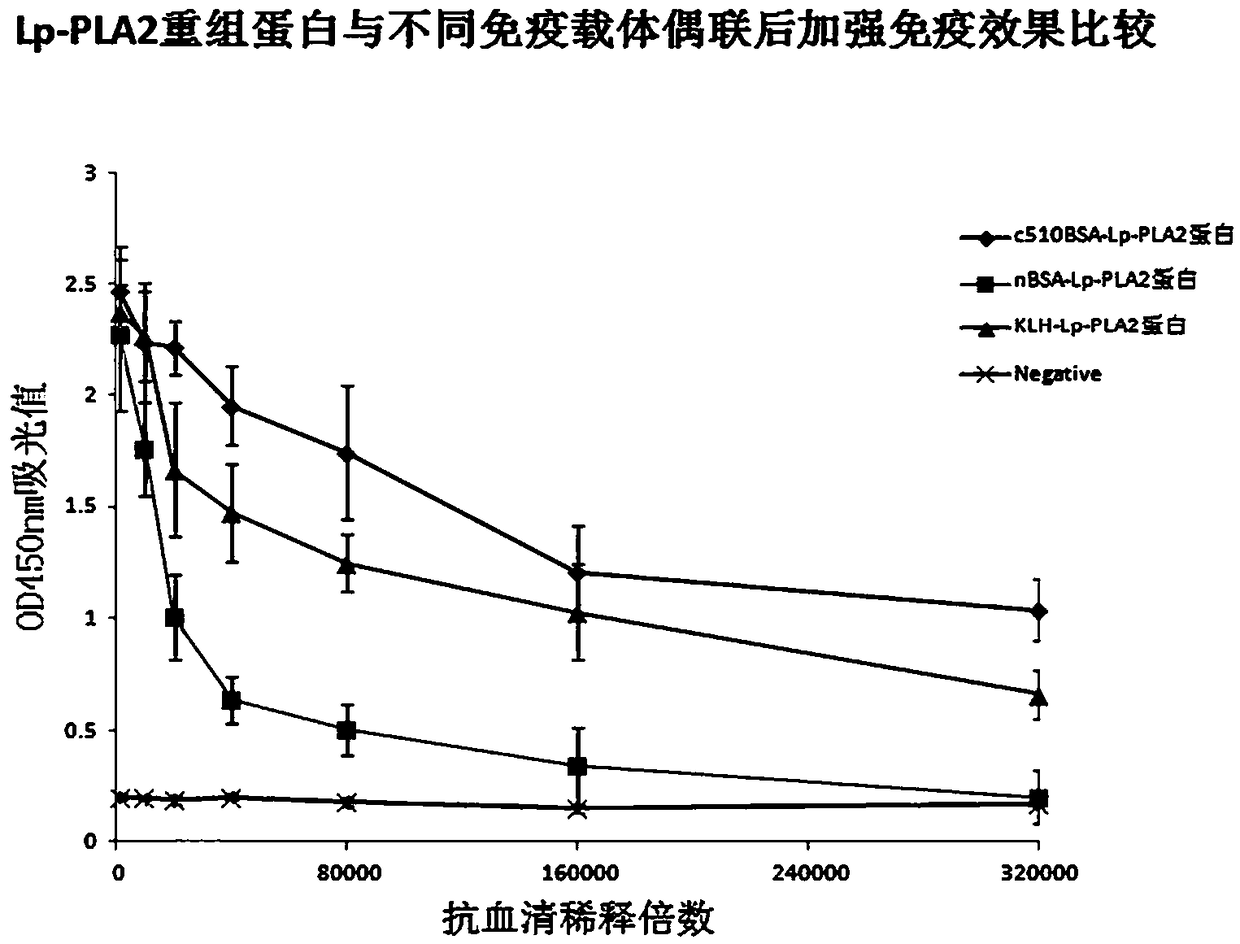
[0062] Example 3: The immune effect of the c510BSA-LP-PLA2 protein immunogen conjugate
[0063] (1), the prepared immunization antigen is the c510BSA-LP-PLA2 recombinant protein immunogen conjugate prepared in Example 2, the conjugate nBSA-LP-PLA2 of natural bovine serum albumin (nBSA) and LP-PLA2 recombinant protein , and the conjugate KLH-LP-PLA2 of hemocyanin (KLH) and LP-PLA2 recombinant protein;
[0064] (2), using the c510BSA-LP-PLA2, nBSA-LP-PLA2 and KLH-LP-PLA2 immunogens prepared in Example 2, adopt conventional methods to inoculate experimental animal mice (BALB / c) respectively, and take small mice after booster immunization. Mouse antiserum, the specific steps are as follows:
[0065] Dilute the c510BSA-LP-PLA2, nBSA-LP-PLA2 and KLH-LP-PLA2 immunogens synthesized above to 1 mg / ml with sterile water to obtain an antigen solution, and then use 1.0 ml of the antigen solution with an equal amount of Freund’s After the complete adjuvant was mixed, the experimental mice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com