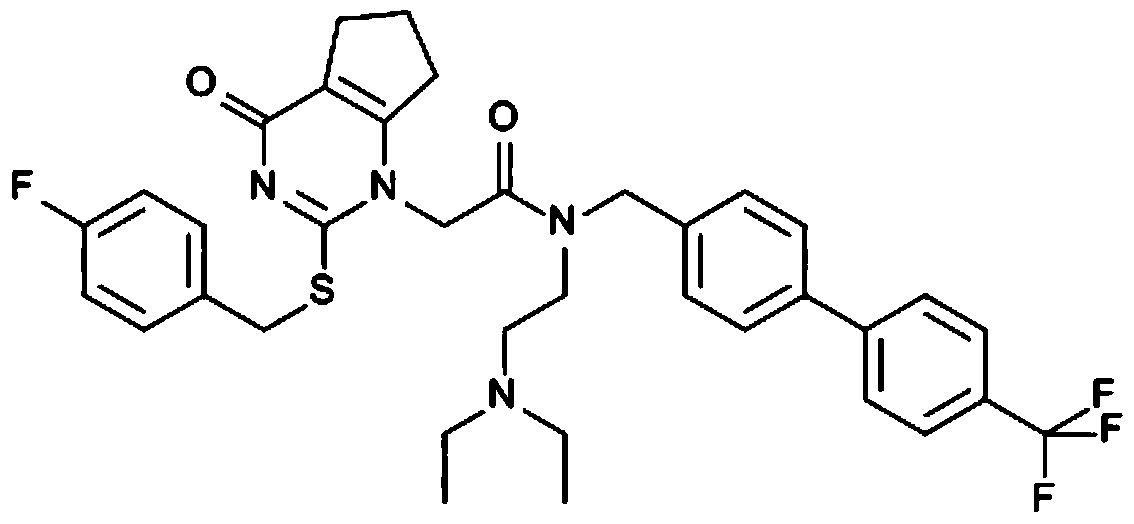
Pharmaceutical composition and application of lipoprotein-related phospholipase a2 inhibitor
A correlation, phospholipase technology, applied in the field of medicine, can solve problems such as poor myocardial infarction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
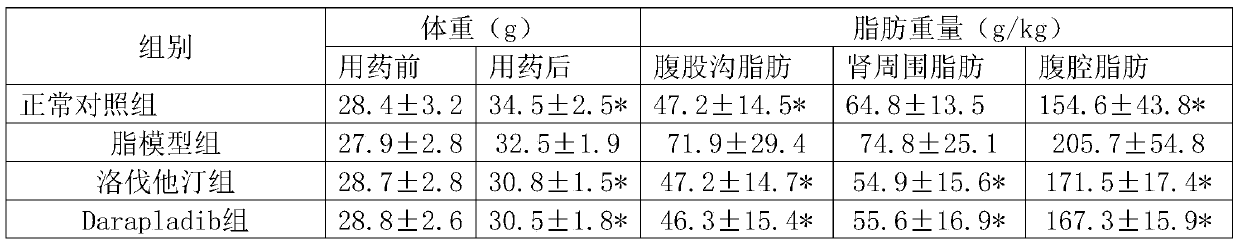
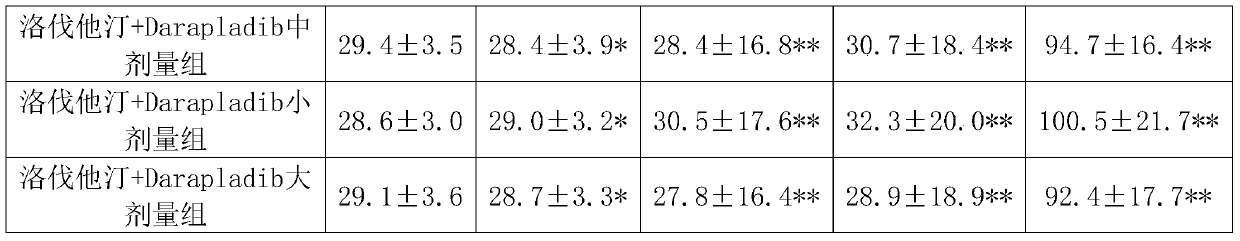
[0050] Experimental Example 1: Pharmacological effects of lovastatin + Darapladib
[0051] 1. Animals and grouping 105 healthy NIH 8-week-old male mice were randomly divided into 7 groups, 15 in each group, of which:
[0052] Group 1 was the normal control group, fed with distilled water;
[0053] Group 2 was the high-fat model group, which was given high-cholesterol emulsion by intragastric administration;
[0054] Group 3 was the lovastatin group, which received lovastatin 4 mg / kg orally;
[0055] Group 4 is the Darapladib group, and Darapladib 30mg / kg is administered orally;
[0056] Group 5 was lovastatin+Darapladib middle-dose group: lovastatin 4mg / kg and Darapladib 30mg / kg by intragastric administration.
[0057] Group 6 was lovastatin+Darapladib low-dose group: lovastatin 2mg / kg and Darapladib 20mg / kg by intragastric administration.
[0058] Group 7 was the lovastatin+Darapladib high-dose group: lovastatin 6 mg / kg and Darapladib 40 mg / kg by intragastric administrati...
Embodiment 2
[0070] Example 2: Darapladib and lovastatin tablets
[0071] 1. Prescription (1000 tablets)
[0072] composition
Weight (g)
percentage (%)
Darapladib
80
31.68
10
3.96
100
39.60
Crospovidone
4
1.58
50
19.80
Povidone
6
2.38
50
—
2.5
0.99
[0073] total
252.5
100.00
[0074] Remarks: Water used as a solvent evaporates when it is finally dried, and is not included in the prescription composition, so it is not counted.
[0075] 2. Preparation process
[0076] 1) Weigh each component according to the prescription, take Darapladib, lovastatin, microcrystalline cellulose, crospovidone and lactose, mix them evenly, and set aside.
[0077] 2) Take the prescribed amount of povidone and dissolve it in an appropriate amount of purified water to ma...
experiment example 3
[0079] Experimental example 3 Pharmacological effects of fenofibrate + Darapladib
[0080] 1. Animals and Grouping A total of 70 NIH mice, half male and half male, were divided into 7 groups with 10 mice in each group, of which:
[0081] Group 1 is the normal control group, NIH 5-month-old non-modeled normal mice;
[0082] Groups 2, 3, 4, 5, 6, and 7 were 5-month-old obese mice, and group 2 was the model control group; group 3 was the fenofibrate group, and 50 mg / kg; Group 4 was Darapladib group, Darapladib 26 mg / kg orally; Group 5 was fenofibrate + Darapladib medium dose group, fenofibrate + Darapladib 76 mg / kg orally, of which fenofibrate 50 mg / kg ; Group 6 was fenofibrate + Darapladib low-dose group, fenofibrate + Darapladib 60mg / kg by intragastric administration, of which fenofibrate 40mg / kg; Group 7 was fenofibrate + Darapladib high-dose group, intragastric administration Fenofibrate + Darapladib 90mg / kg, of which fenofibrate 60mg / kg, after continuous gavage for 1 mont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com