A compound pharmaceutical composition of glandularin and andrographolide and its application
A technology of andrographolide and adenophyllin, which is applied in the direction of drug combination, pharmaceutical formula, antineoplastic drugs, etc., can solve the problems that the effects have not been reported yet, achieve good clinical drug development prospects, strong anti-proliferation activity, and reduce toxic and side effects the effect produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
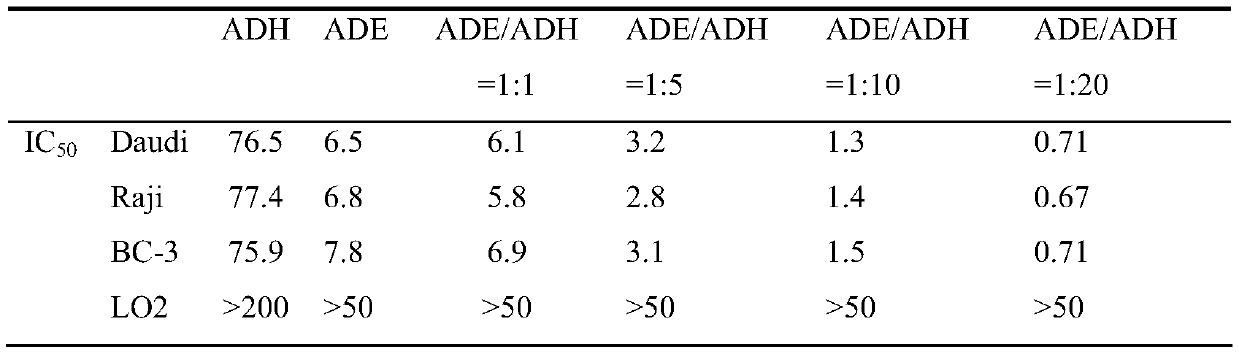
[0021] Embodiment 1: adopt Daudi cell, Raji cell and BC-3 cell to screen and optimize adenflowerin (ADE) and andrographolide (ADH) compound pharmaceutical composition
[0022] Take the cells in the logarithmic growth phase and inoculate 3×10 4 Cells / well were placed on a 96-well plate. After 6 hours of growth, the supernatant was discarded by centrifugation, and then administered according to the following groups: Tumor cells were divided into no drug group and drug-added group, and the drug-added group was divided into ADH and ADE single drugs. group, ADH and ADE combined drug group with different molar ratios, set 4-6 duplicate wells in each group, culture for 24 hours, discard the supernatant, add 100 μl of MTT (tetrazolium salt) serum-free culture medium containing 0.5 mg / ml for culture After 4 hours, add 100 μl of DMSO (dimethyl sulfoxide), place on a micro-oscillator to vibrate for 10 minutes, and then place on a microplate reader to detect the OD value at 570 nm. Resul...
Embodiment 2
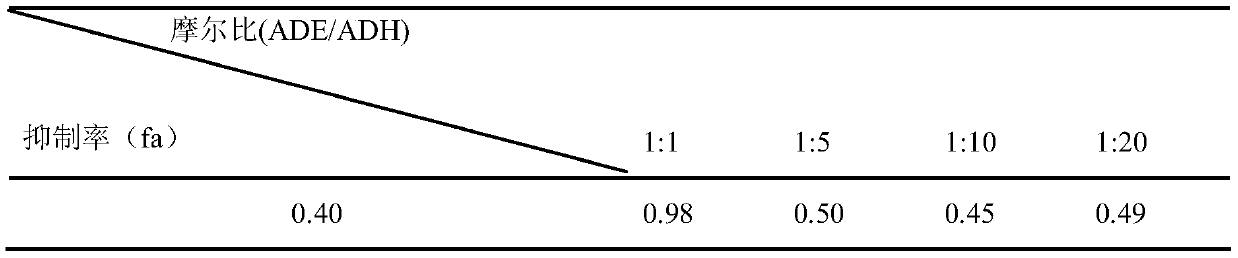
[0028] Embodiment 2: Analysis of the combined administration effect of ADE and ADH
[0029] Based on the Median-effect Principle (moderate effect principle or Chou-Talalay combined index method), the dose-response curve and the combined index curve (fa-C curve) under different effects were drawn using CombiDrug statistical software. The relationship between the effect and the combination index quantitatively evaluates whether there is a synergistic, antagonistic or additive relationship between the two drugs. Specific steps are as follows:
[0030] The drug effect is the inhibition rate (fa)=1-(the average OD570 value of the test group / the average OD570 of the tumor cell blank control group) according to the intermediate effect equation fa / fu=(D / Dm) m , take the logarithm logfa / fu=mlogD–mlogDm on both sides, set a=-mlogDm, b=m, x=logD, y=logfa / fu, and substitute into the intermediate effect equation to get y=bx–a; where fa is the drug effect , fu=1–fa, D is the drug concentr...
Embodiment 3
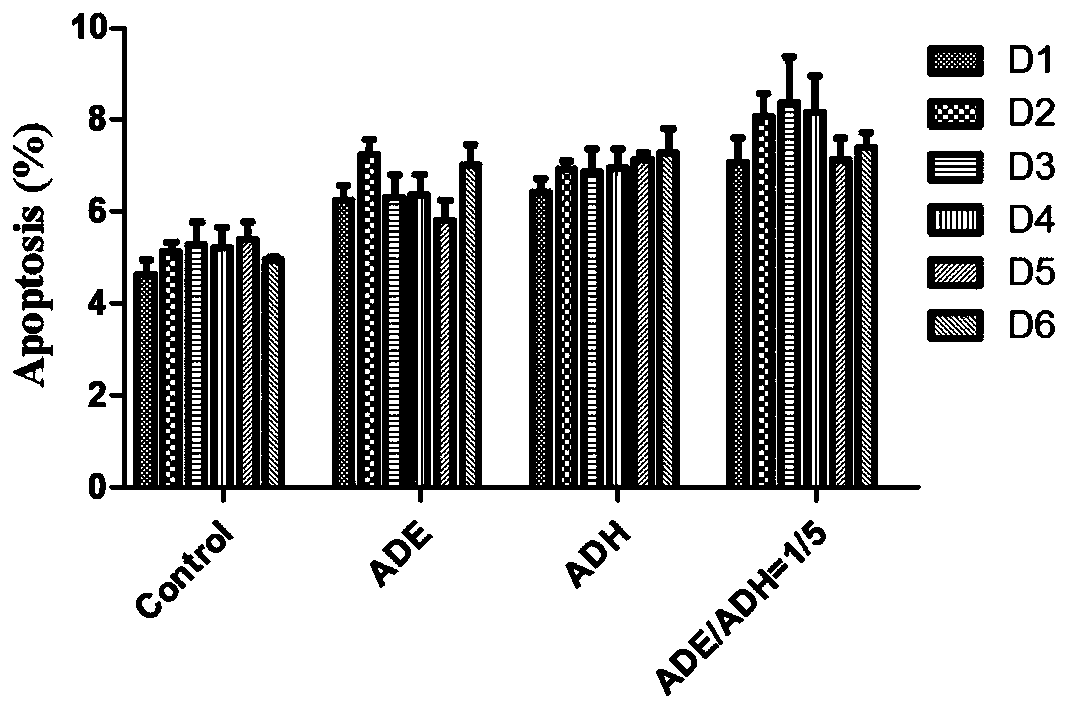
[0036] Example 3: Safety of ADE and ADH on peripheral blood mononuclear lymphocytes
[0037]The safety of ADH and ADE on PBMC cells was evaluated after isolation and culture of mononuclear lymphocytes from healthy volunteers ( figure 1 ): ① draw fresh blood from healthy volunteers into a heparin anticoagulant tube (sterile); then resuspend the cells (sterile) with an equal volume of PBS (or serum-free D-Hank buffer); ② put the suspended cells Add the pre-coated human lymphocyte separation medium (preheated at 37°C), the volume ratio of lymphatic separation medium to cell suspension is not less than 1:1; ③ 500×g (or 2000rpm) horizontal centrifuge for 20-30min, room temperature (20-30°C) at slow speed; ④Discard the upper layer of plasma, carefully suck out the middle white mist layer and add it to 5ml (or 1-2 times the volume) of PBS (or serum-free cell culture buffer), 200×g or 1000rpm Centrifuge for 10 minutes at room temperature (20-30°C) at a slow speed, discard the superna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com