Multi-arm PEGylated (Polyethylene Glycolylated) eliglustat derivative and preparation thereof
A technology for synthesizing ilulustat and derivatives, which is applied in the field of multi-arm PEGylated ilulustat derivatives and their preparation, can solve problems such as limited loading, achieve good water solubility, improve curative effect, and increase the existence time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
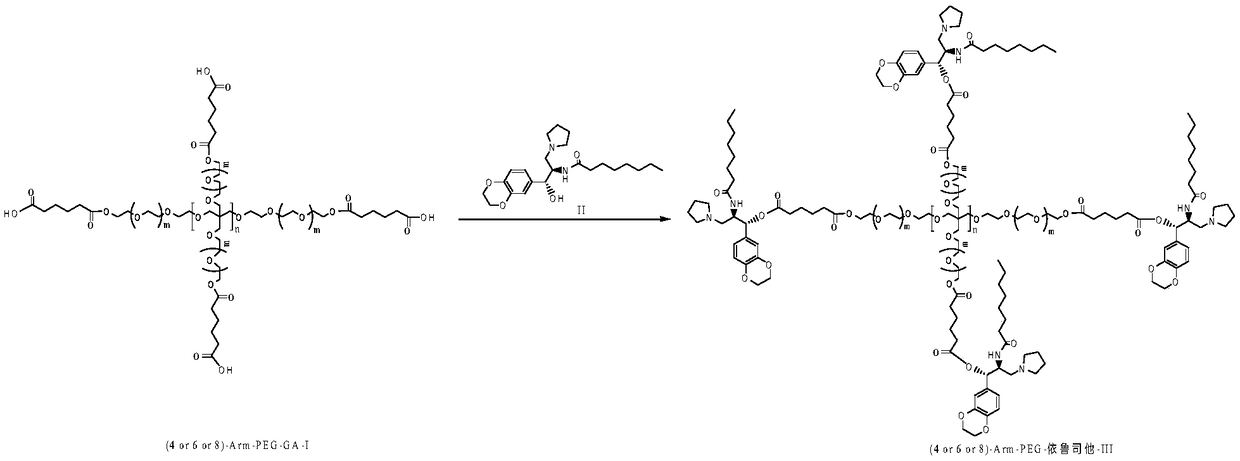
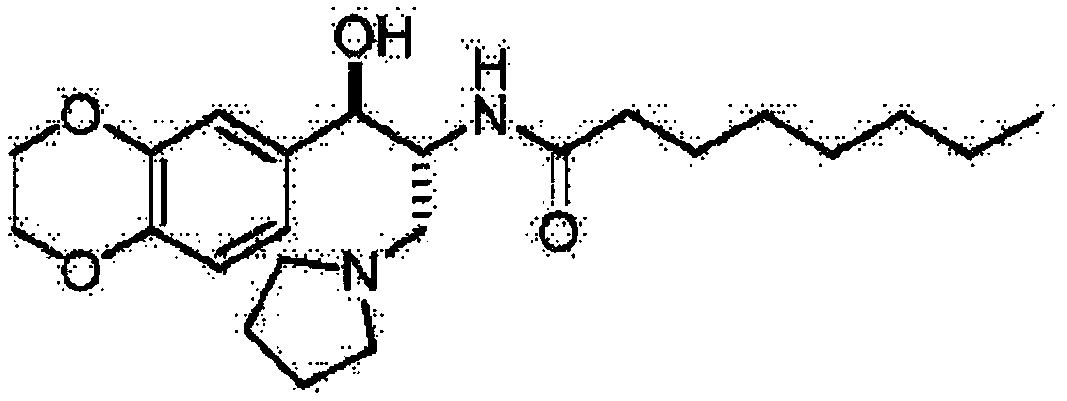
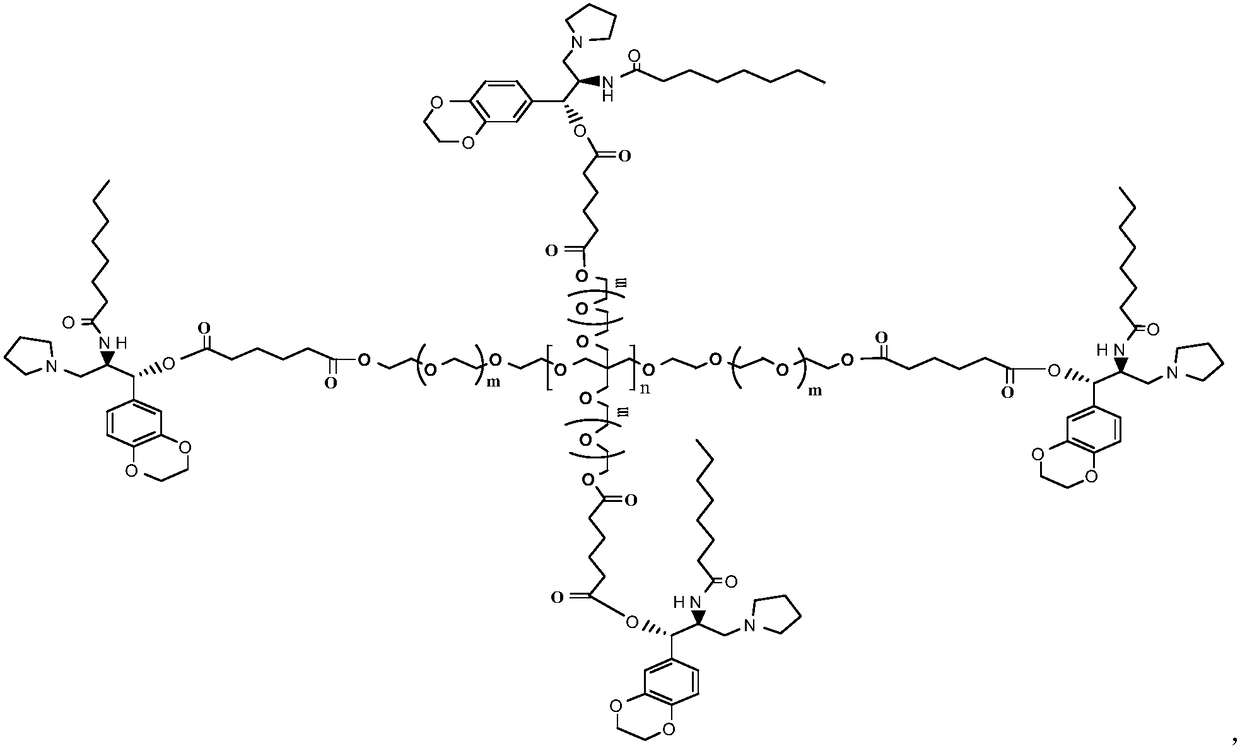
[0025] Preparation of 4arm-PEG24-Ebrustat (III)
[0026] Dissolve 10mmol of 4-Arm-PEG24-GA-I in 50ml of N,N-dimethylformamide, add 10mmol of EDCI and 10mmol of NHS, and stir at 25°C for 2h. 2 mmol of 4arm-PEG24-NH2 was added to the reaction, and the reaction was carried out at 30°C for 12 hours. After the complete reaction of ebrustat was detected by TLC tracking, 9.4 mmol of 4arm-PEG24-elukastat (I) was obtained by recrystallization and column chromatography. Yield: 94%. NMR data are as follows: HNMR(CDCl3)δ6.72-6.88(m,3H),5.80(d,1H),4.88(d,1H),4.24-4.21(m,6H),4.14-4.21(m,1H) ,3.63(s, 96H),2.72-2.83(m,4H),2.53-2.70(m,6H),2.07(t,2H),2.04(s,2H),1.85-1.74(m,4H),1.44 -1.57(m,2H),1.14-1.31(m,8H),0.87(t,3H).
Embodiment 2
[0028] Preparation of 4arm-PEG124-Ebrustat (III)
[0029] Dissolve 10mmol of 4-Arm-PEG124-GA-I in 50ml of N,N-dimethylformamide, add 10mmol of DCC and 6mmol of DMAP, and stir at 25°C for 2h. 2 mmol of 4arm-PEG124-NH2 was added to the reaction, and the reaction was carried out at 25°C for 16 hours. After the completion of the reaction of ebrustat by TLC tracking detection, the pure 4arm-PEG124-elukastat (III) was obtained by recrystallization and chromatography column purification. Yield: 90%. NMR data are as follows: HNMR(CDCl3)δ6.72-6.88(m, 3H), 5.80(d,1H), 4.88(d,1H), 4.24-4.21(m,6H), 4.14-4.21(m,1H) ,3.63(s, 496H),2.72-2.83(m,4H),2.53-2.70(m,6H),2.07(t,2H),2.04(s,2H),1.85-1.74(m,4H),1.44 -1.57(m,2H),1.14-1.31(m,8H),0.87(t,3H).
Embodiment 3
[0031] Preparation of 4arm-PEG240-Ebrustat (III)
[0032] Dissolve 10mmol of 4-Arm-PEG240-GA-I in 50ml of dichloromethane, add 10mmol of HOBT and 8mmol of DIC, and stir at 25°C for 2h. 2 mmol of 4arm-PEG240-NH2 was added to the reaction, and the reaction was carried out at 30°C for 20 hours. After the completion of the reaction of ebrustat through TLC tracking detection, the pure 4arm-PEG240-elukastat (III) was obtained by recrystallization and chromatographic column purification. Yield: 86.9%. NMR data are as follows: HNMR(CDCl3)δ6.72-6.88(m, 3H), 5.80(d,1H), 4.88(d,1H), 4.24-4.21(m,6H), 4.14-4.21(m,1H) ,3.63(s, 960H),2.72-2.83(m,4H),2.53-2.70(m,6H),2.07(t,2H),2.04(s,2H),1.85-1.74(m,4H),1.44 -1.57(m,2H),1.14-1.31(m,8H),0.87(t,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com