Foaming formula for nasal care
A formula and nasal cavity technology, applied in the field of care agents, can solve problems such as delayed disintegration time, poor solubility, and decreased stability of dosage forms, and achieve the effect of solving compatibility problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
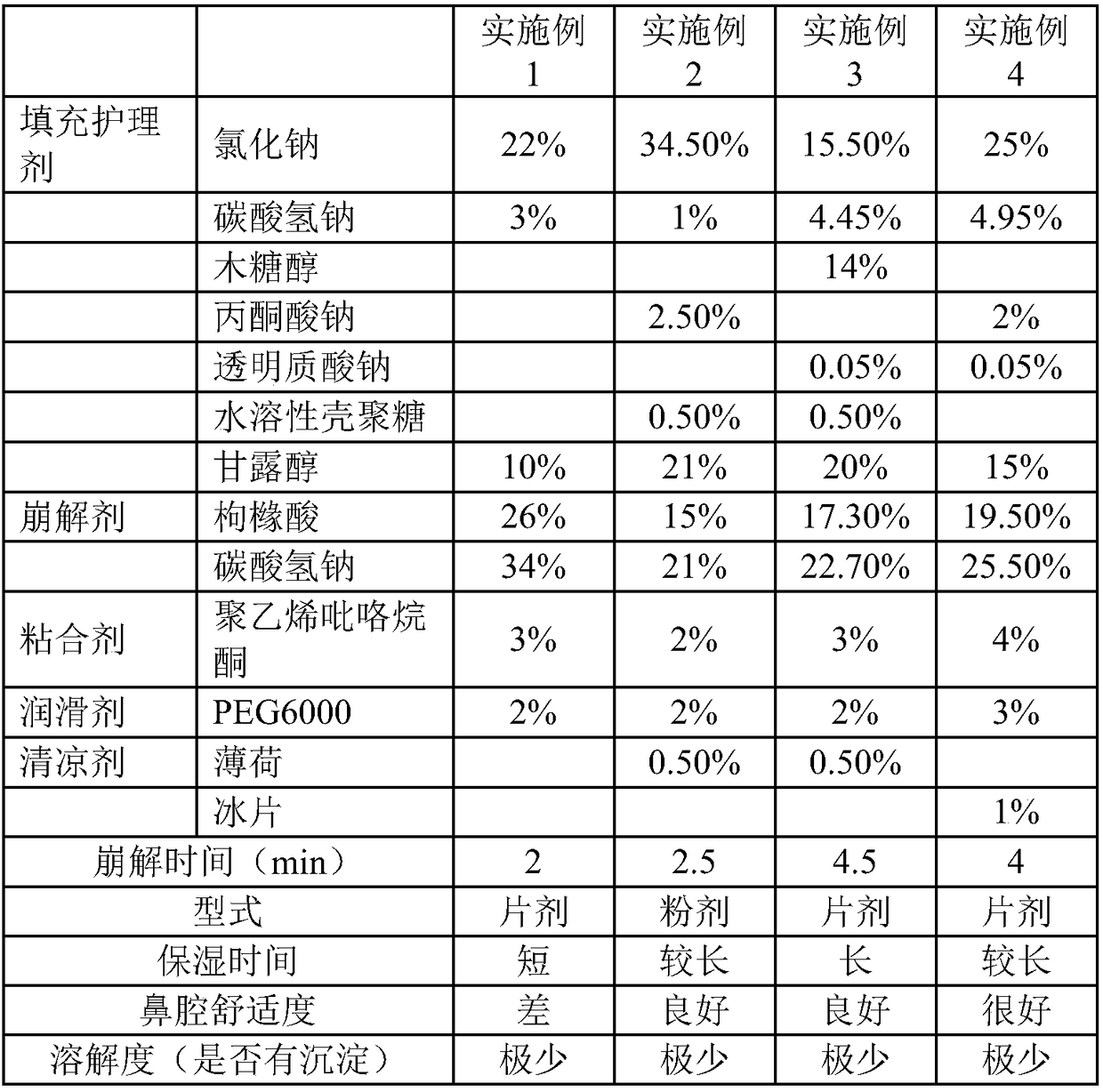
[0028] An effervescent formula for nasal cavity care, the specific weight percentage of each component is: filling care agent, including 22% sodium chloride, 3% sodium bicarbonate and 10% mannitol; disintegrating agent, including 26% citric acid and 34% sodium bicarbonate; binder, including 3% polyvinylpyrrolidone; lubricant, including 2% PEG6000. The disintegration time of the prepared tablet is 2 minutes, the moisturizing time is short, the nasal cavity comfort is poor, and there is very little precipitation.
Embodiment 2
[0030] An effervescent formula for nasal cavity care, the weight percentage of each component is specifically: filling care agent, including 34.5% sodium chloride, 1% sodium bicarbonate, 2.5% sodium pyruvate, 0.5% water-soluble Chitosan and 20% mannitol; disintegrants, including 15% citric acid and 21% sodium bicarbonate; binders, including 2% polyvinylpyrrolidone; lubricants, including 2% PEG6000 ; Cooling agents, including peppermint 0.5%. The disintegration time of the prepared powder is 2.5 minutes, the moisturizing time is long, the nasal cavity is comfortable, and there is very little precipitation.
Embodiment 3
[0032] An effervescent formula for nasal cavity care, the specific weight percentage of each component is: filling care agent, including 15.5% sodium chloride, 4.45% sodium bicarbonate, 14% xylitol, 0.05% transparent Sodium hyaluronate, 0.5% water-soluble chitosan, and 20% mannitol; disintegrant, including 17.3% citric acid and 22.7% sodium bicarbonate; binder, including 3% polyvinylpyrrolidone ; lubricants, including 2% PEG6000; cooling agents, including 0.5% mint. The disintegration time of the prepared tablet is 4.5 minutes, the moisturizing time is long, the nasal cavity is comfortable, and there is very little precipitation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com