Method for catalyzing etherification of benzyl alcohol compounds
A technology for catalyzing benzyl alcohol and compounds, which is applied in the dehydration of hydroxyl-containing compounds to prepare ether, ether preparation, organic chemistry, etc., can solve the problem of high cost of chemical pollution, and achieve the effects of high reaction efficiency, high chemical selectivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
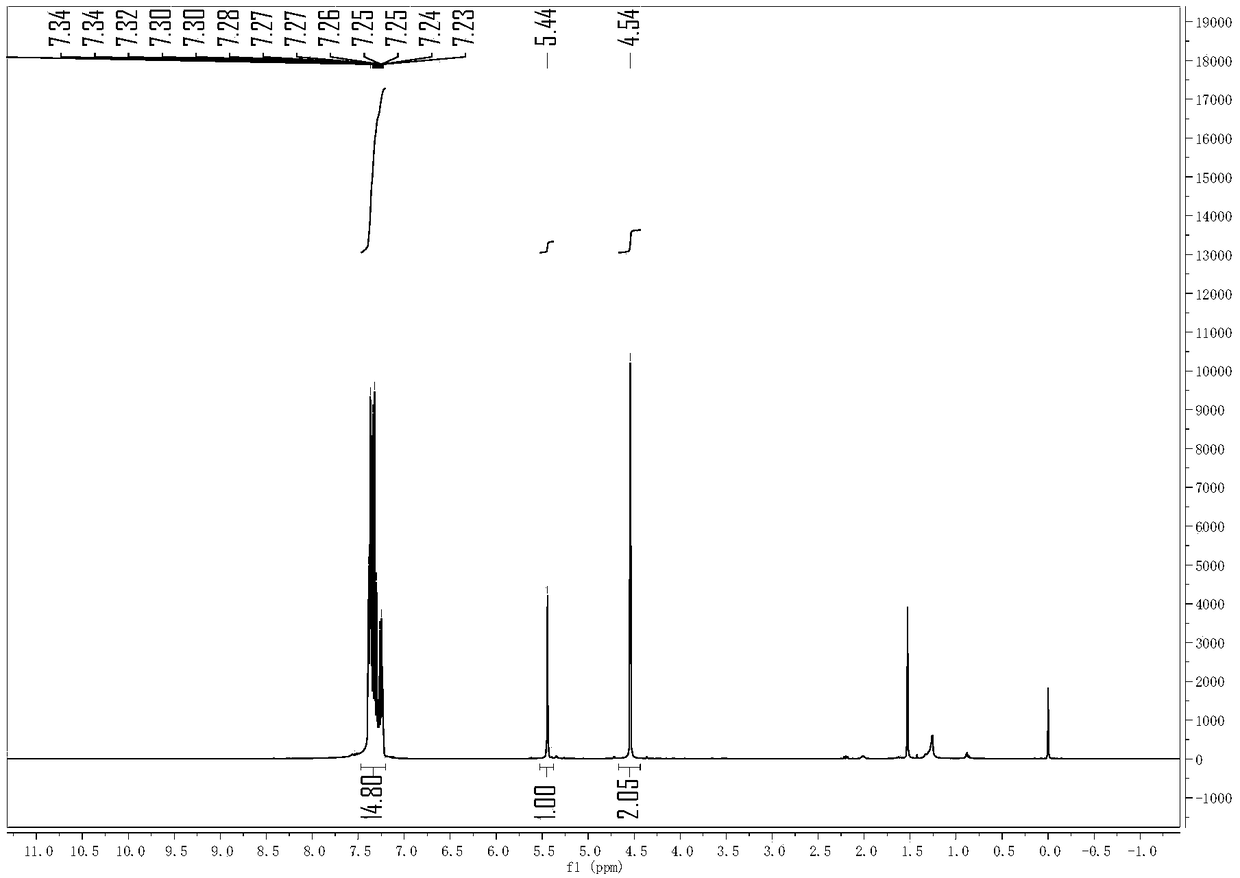
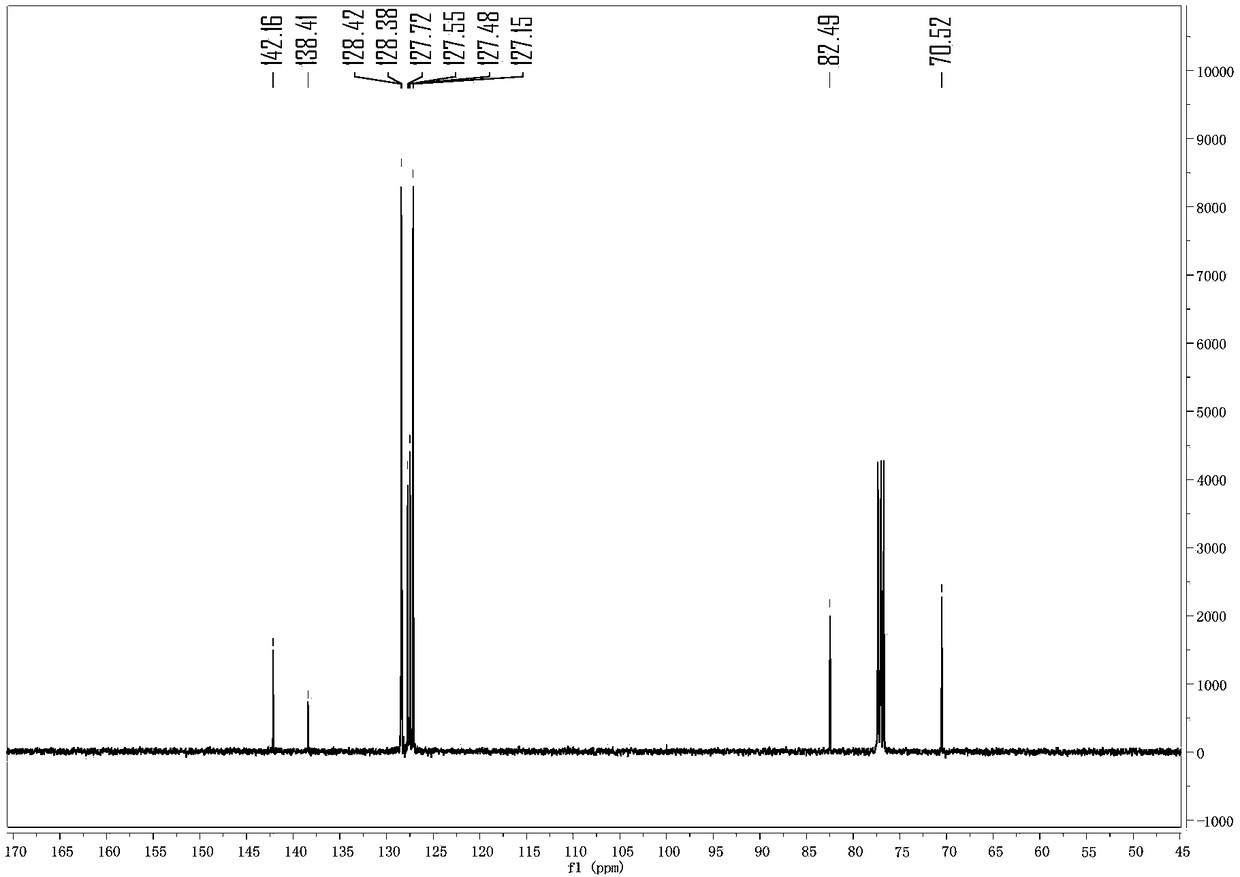
[0031] Put benzhydryl alcohol (36.8 mg) in a 10 ml reaction tube, add 1 ml of 1,2-dichloroethane as a solvent, then add 11 mg of tripentafluorophenylborane and benzyl alcohol (43.2 mg), and the reaction temperature Raise to 60°C, react for 4 hours, then cool the reaction to room temperature, then remove the solvent and separate the product by column chromatography: benzhydryl benzyl ether 52 mg, 95% yield, colorless liquid. analyze data: 1 H NMR (400MHz, Chloroform-d) δ7.55–6.85 (m, 15H), 5.44 (s, 1H), 4.54 (s, 2H). 13 C NMR(100MHz,Chloroform-d)δ142.16,138.41,128.42,128.38,127.72,127.55,127.48,127.15,82.49,70.52.HRMS(ESI)m / z[M+Na] + :Calcd forC20H18ONa:297.1255.Found:297.1253.
Embodiment 2
[0033]
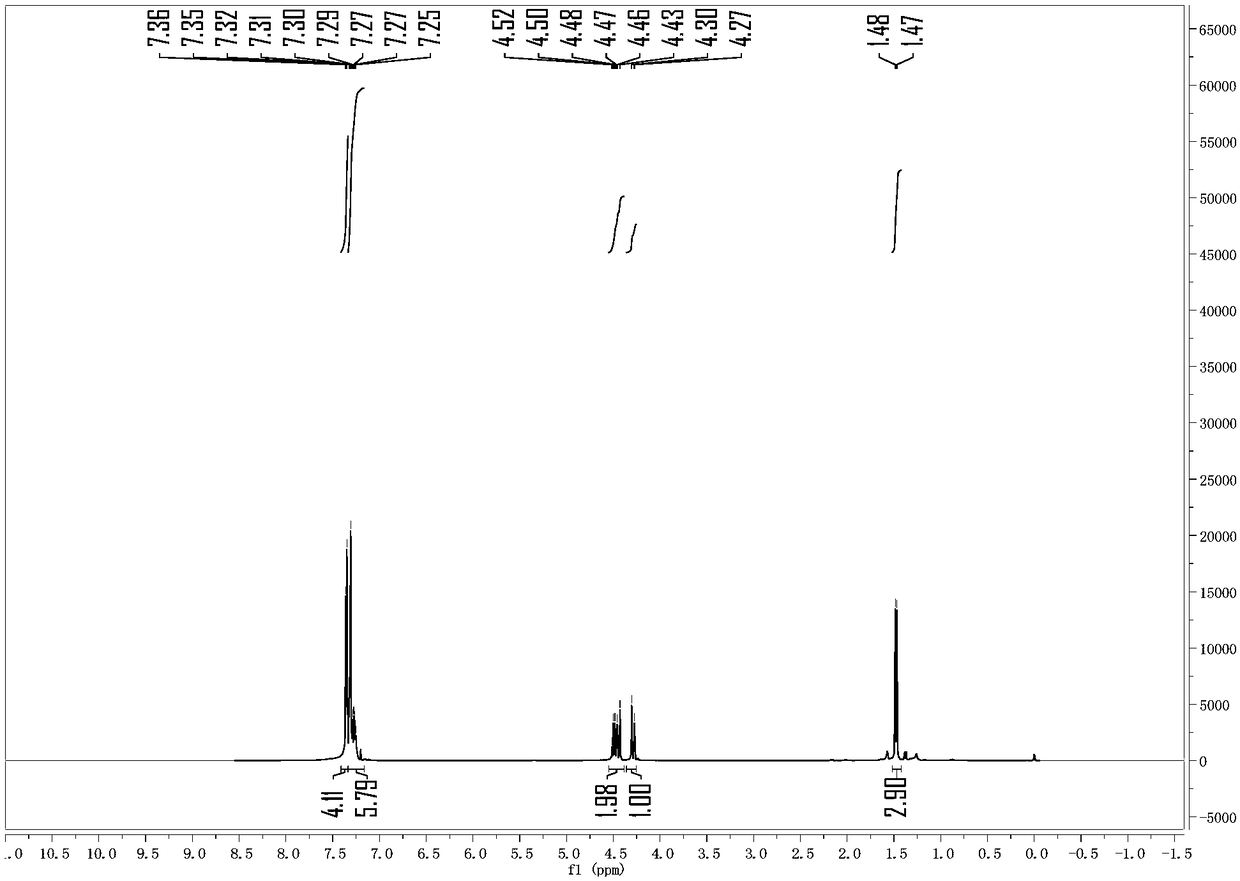
[0034] 1-phenylethanol (24.4mg) was placed in a 10ml reaction tube, 1ml of 1,2-dichloroethane was added as a solvent, followed by 11mg of tripentafluorophenylborane and benzyl alcohol (43.2mg), The reaction temperature was raised to 60°C. After 12 hours of reaction, the reaction was cooled to room temperature and the solvent was removed and the product was isolated by column chromatography: 1-phenethyl benzyl ether 40.7 mg, 96% yield, colorless liquid. analyze data: 1 H NMR (400MHz, Chloroform-d) δ7.35 (d, J = 4.4Hz, 4H), 7.29 (m, 6H), 4.57–4.39 (m, 2H), 4.29 (d, J = 11.9Hz, 1H) ,1.48(d,J=6.5Hz,3H). 13 C NMR (100MHz, Chloroform-d) δ143.78, 138.70, 128.55, 128.40, 127.74, 127.55, 127.51, 126.39, 77.28, 70.36, 24.27. HRMS (ESI) m / z [M+Na] + :Calcd for C15H16ONa:235.1099.Found:235.1098.
Embodiment 3
[0036]
[0037] Benzyl alcohol (36.8 mg) was placed in a 10 ml reaction tube, 1 ml of 1,2-dichloroethane was added as a solvent, followed by 11 mg of tripentafluorophenylborane and isopropanol (24.6 mg), and the reaction The temperature was raised to 60°C, and after 4 hours of reaction, the reaction was cooled to room temperature and the solvent was removed and the product was isolated by column chromatography: benzyl alcohol isopropyl ether 43.4 mg, 96% yield, colorless liquid. analyze data: 1 H NMR (400MHz, Chloroform-d) δ7.21(m,10H),5.40(s,1H),3.58(dt,J=12.2,6.1Hz,1H),1.13(d,J=6.1Hz,6H) . 13 C NMR (100MHz, Chloroform-d) δ143.04, 128.37, 127.30, 127.14, 80.52, 69.15, 22.33. HRMS (ESI) m / z [M+Na] + :Calcd for C16H18ONa:249.1255.Found:249.1253.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com