A combination drug therapy composition used for treating amyotrophic lateral sclerosis and a preparation method and use thereof
A technology of lateral sclerosis and drug combination, which is applied in the direction of drug combination, drug formulation, drug delivery, etc., can solve the problems of unsatisfactory effects, and achieve the effect of no toxic side effects and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
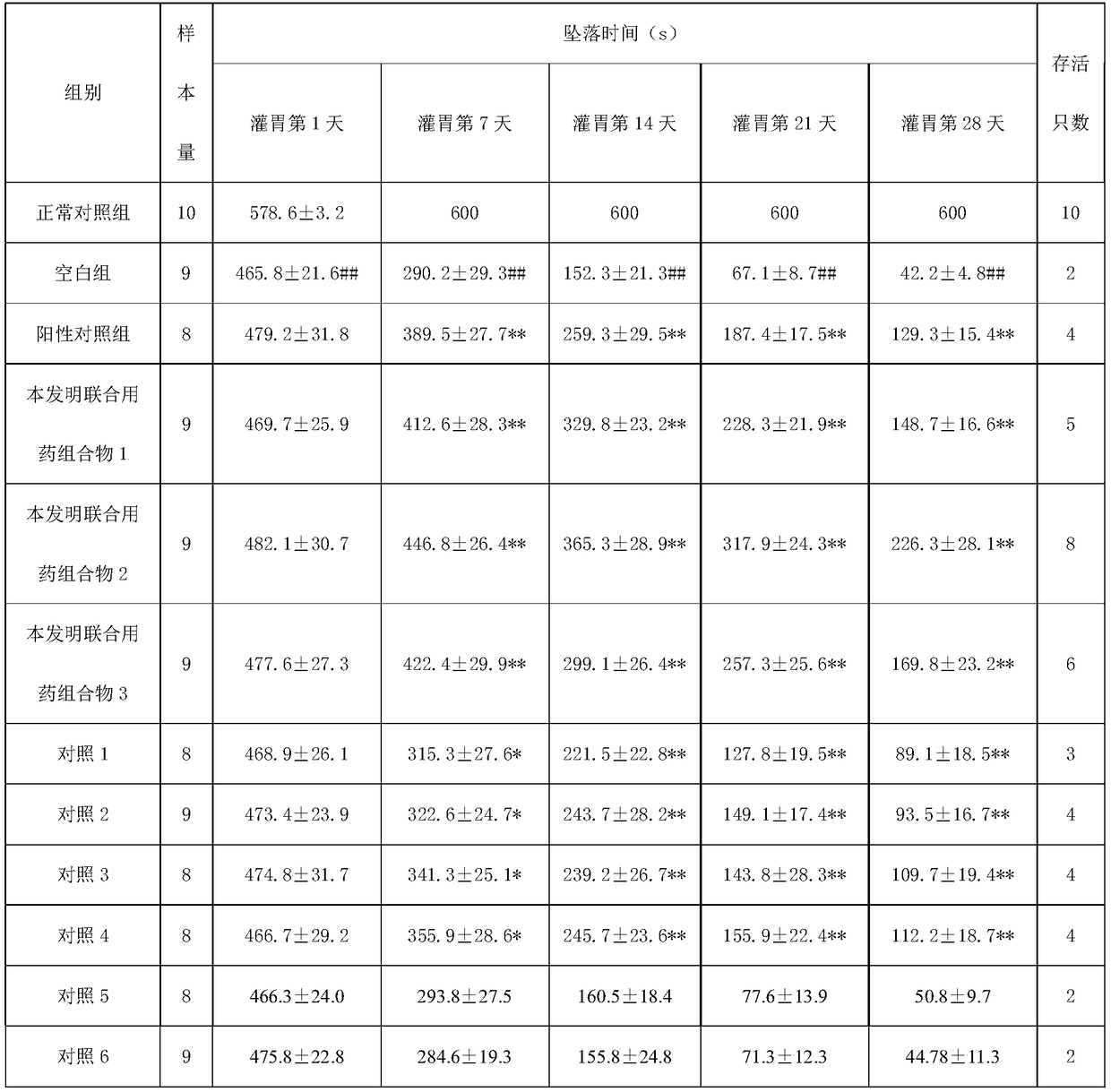
[0032] Embodiment 1: a kind of combination medicine composition for the treatment of amyotrophic lateral sclerosis
[0033] It consists of a unit preparation containing 300 mg of chlorogenic acid, a unit preparation containing 500 mg of vitamin E, a unit preparation containing 600 mg of coenzyme Q10, and a unit preparation containing 2500 mg of creatine phosphate sodium. The unit preparations in the combined drug composition are administered simultaneously or separately medicine.
Embodiment 2
[0034] Embodiment 2: A kind of tablet for treating amyotrophic lateral sclerosis
[0035] Chlorogenic acid 300g, vitamin E 500g, vitamin C 600g, coenzyme Q10 600g, creatine phosphate sodium 2500g, prepared according to the following method:
[0036] (1) Material preparation: take each bulk drug according to the formula;
[0037] (2) Mixing: Mix chlorogenic acid, vitamin E, vitamin C, coenzyme Q10 and creatine sodium phosphate evenly, add microcrystalline cellulose, hydroxypropyl methylcellulose, sodium carboxymethyl starch, and then wet granulate, After adding magnesium stearate, tableting was made into 10,000 tablets, each containing 30 mg of chlorogenic acid, to obtain the tablet for treating amyotrophic lateral sclerosis of the present invention.
Embodiment
[0038] Embodiment: 3, a kind of capsule for the treatment of amyotrophic lateral sclerosis
[0039] Chlorogenic acid 180g, vitamin E 300g, vitamin C 300g, coenzyme Q10 300g, sodium creatine phosphate 1000g, prepared according to the following method:
[0040] (1) Material preparation: take each bulk drug according to the formula;
[0041] Mixing: Mix chlorogenic acid, vitamin E, vitamin C, coenzyme Q10 and creatine sodium phosphate evenly, then add microcrystalline cellulose, hydroxypropyl methylcellulose, sodium carboxymethyl starch, then wet granulate, add stearin Fill it with magnesium acid, and make 6000 capsules in gelatin capsule shells, each containing 30 mg of chlorogenic acid, to obtain the capsule for treating amyotrophic lateral sclerosis of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com