A kind of reversible photochromic material with haloapatite structure and its preparation method and application
A technology of photochromic materials and haloapatite, which is applied in the directions of color-changing fluorescent materials, luminescent materials, chemical instruments and methods, etc., can solve the problem of less inorganic photochromic materials, and achieve good fatigue resistance, cheap and easy raw materials. The effect of obtaining and preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
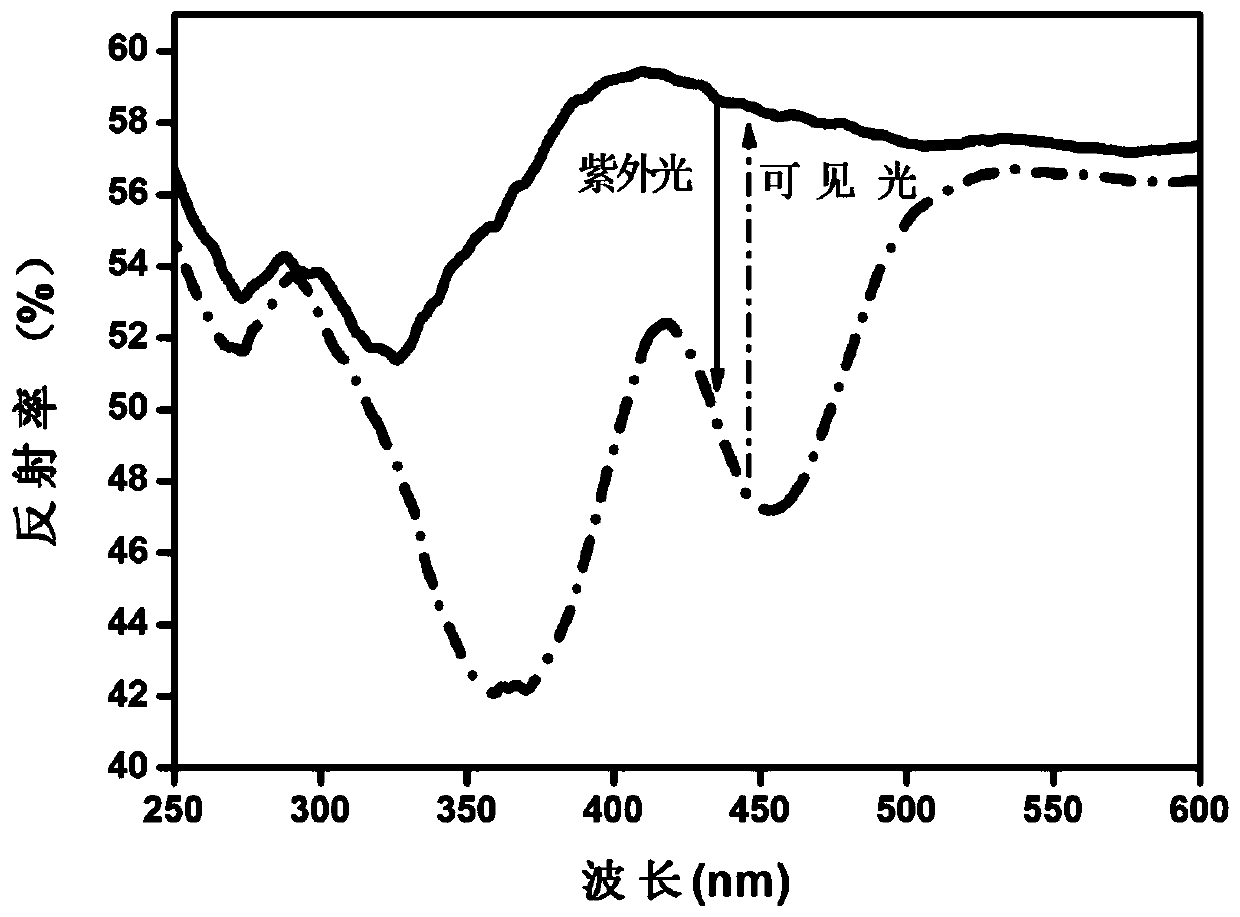
[0063] According to CaCO 3 , CaF 2 , (NH 4 ) 2 HPO 4 , Y 2 o 3 、GeO 2 and Eu 2 o 3 The molar ratio is 7.498: 1.5: 5: 0.5: 1: 0.002. Weigh the above raw materials, mix them and grind them for the first time, put them into the corundum ark after grinding evenly, and heat up in the muffle furnace under the air range first. To 600°C, perform the first constant temperature firing for 3 hours, and then perform the second grinding after natural cooling to room temperature, then raise the temperature to 1420°C in a tube furnace under a weak reducing atmosphere, and carry out the second constant temperature firing for 5 hours. After naturally cooling to room temperature in a tube furnace, the third grinding is carried out to obtain a white fine powder, which is a reverse photochromic material, and its chemical structure is Ca 8.998 Y(PO 4 ) 5 (GeO 4 ) F 2 :0.002Eu 2+ .
[0064] For the diffuse reflectance spectrum detection of the reversible photochromic material prepare...
Embodiment 2
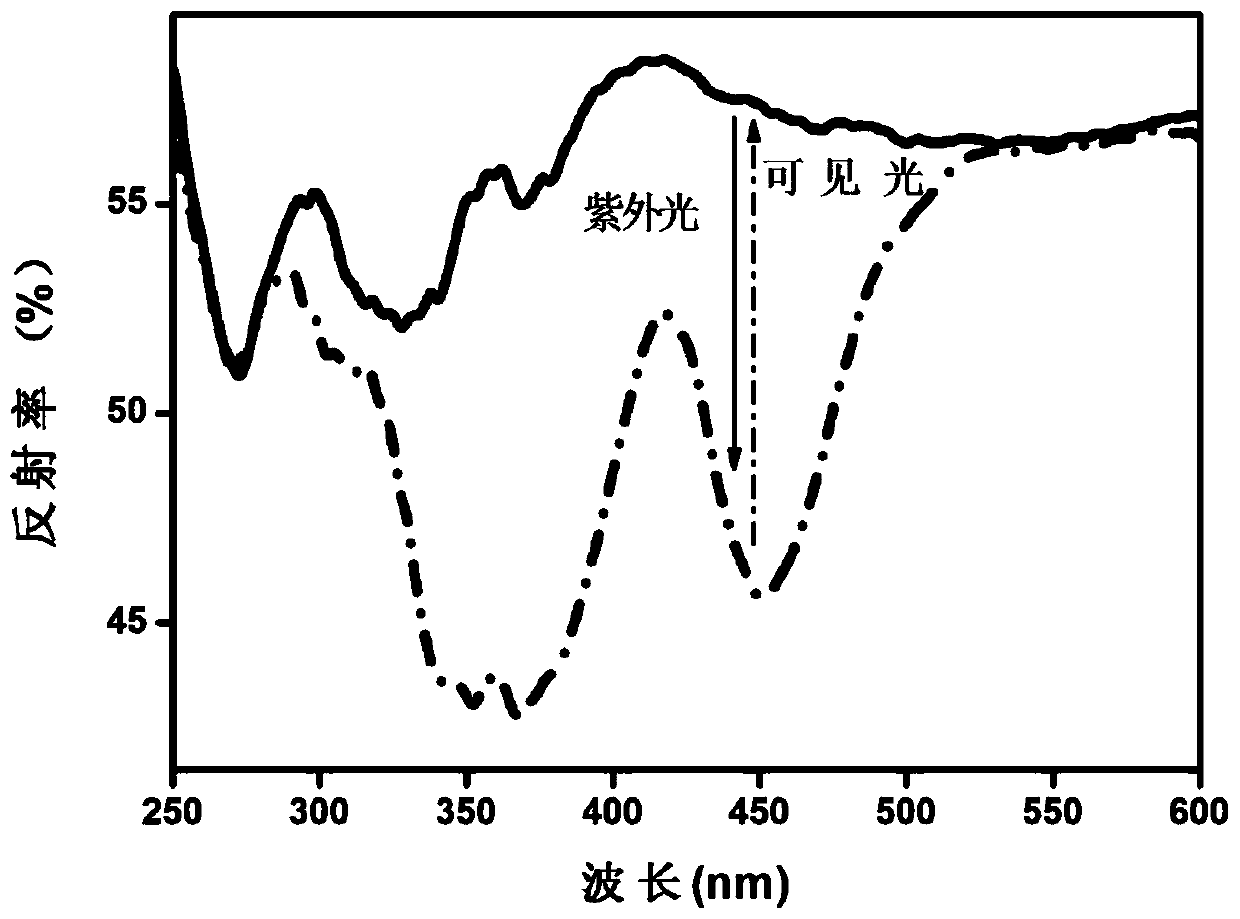
[0066] According to CaCO 3 , CaF 2 , (NH 4 ) 2 HPO 4 , Y 2 o 3 、GeO 2 and Eu 2 o 3 The molar ratio is 7.495: 1.5: 5: 0.5: 1: 0.005. Weigh the above raw materials, mix them and grind them for the first time. Put them into the corundum ark after grinding evenly, and heat up in the muffle furnace under the air range first. To 600°C, perform the first constant temperature firing for 3 hours, and then perform the second grinding after natural cooling to room temperature, then raise the temperature to 1420°C in a tube furnace under a weak reducing atmosphere, and carry out the second constant temperature firing for 5 hours. After naturally cooling to room temperature in a tube furnace, the third grinding is carried out to obtain a white fine powder, which is a reverse photochromic material, and its chemical structure is Ca 8.995 Y(PO 4 ) 5 (GeO 4 ) F 2 :0.005Eu 2+ .
[0067] For the diffuse reflectance spectrum detection of the reversible photochromic material prepare...
Embodiment 3
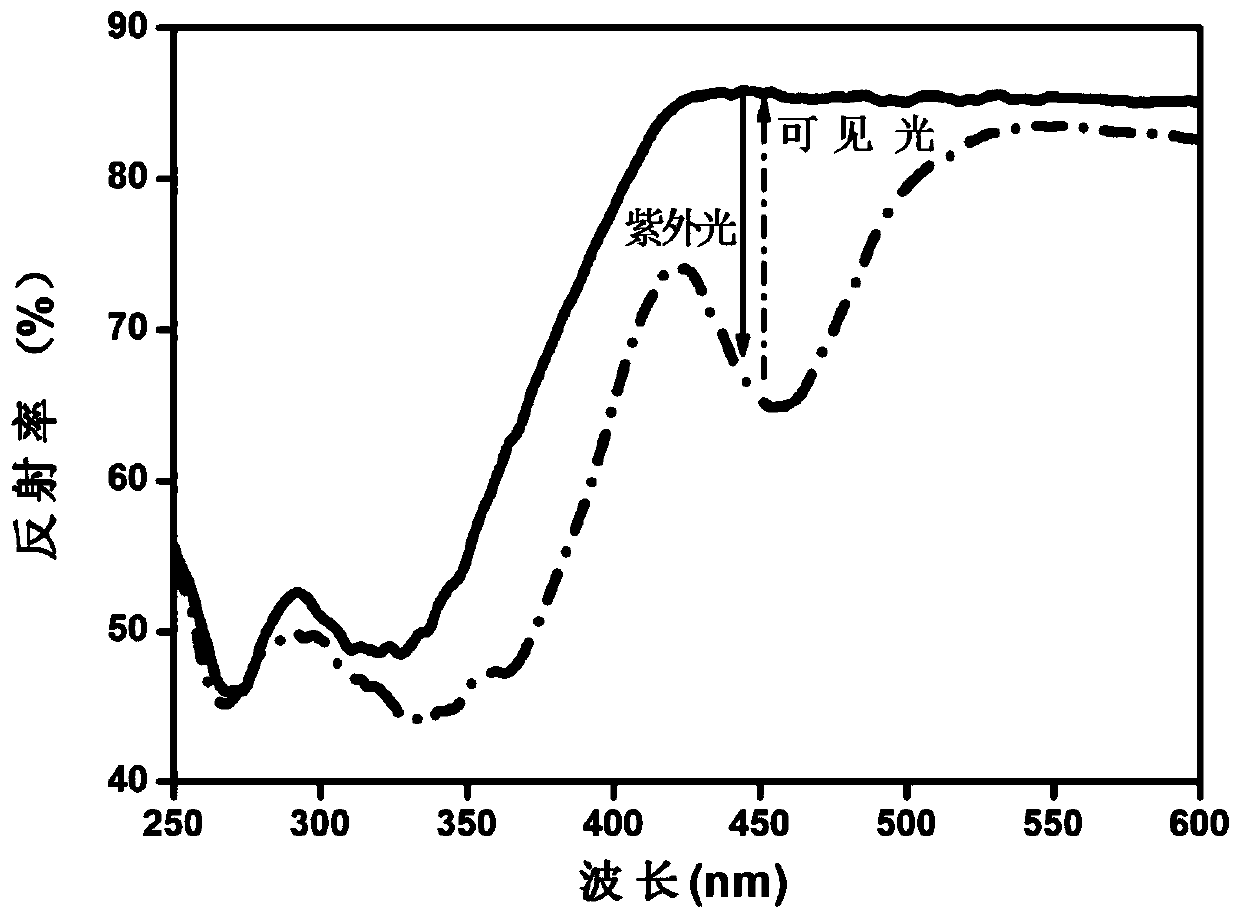
[0069] According to CaCO 3 , CaF 2 , (NH 4 ) 2 HPO 4 , Y 2 o 3 、GeO 2 and Eu 2 o 3 The molar ratio is 7.49: 1.5: 5: 0.5: 1: 0.01. Weigh the above raw materials, mix them and grind them for the first time. Put them into the corundum ark after grinding evenly, and heat up in the muffle furnace under the air range first. To 600°C, perform the first constant temperature firing for 3 hours, and then perform the second grinding after natural cooling to room temperature, then raise the temperature to 1420°C in a tube furnace under a weak reducing atmosphere, and carry out the second constant temperature firing for 5 hours. After naturally cooling to room temperature in a tube furnace, the third grinding is carried out to obtain a white fine powder, which is a reverse photochromic material, and its chemical structure is Ca 8.99 Y(PO 4 ) 5 (GeO 4 ) F 2 :0.01Eu 2+ .
[0070] For the diffuse reflectance spectrum detection of the reversible photochromic material prepared in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com