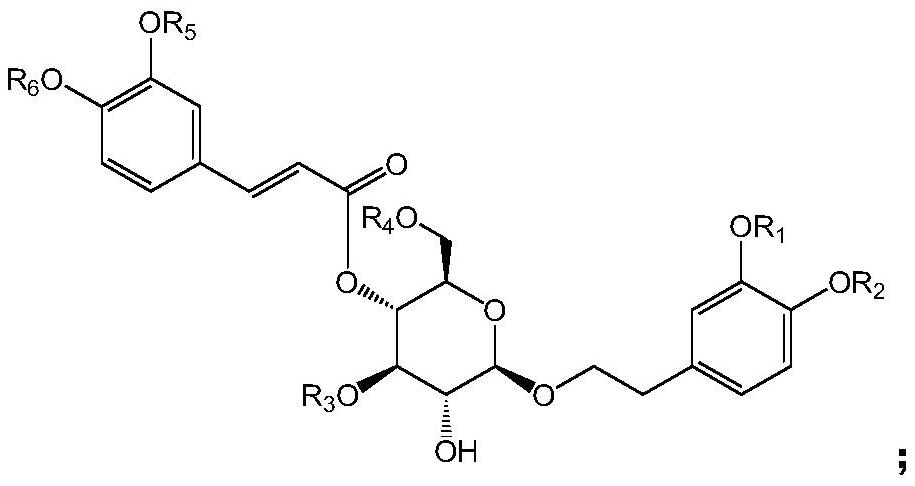
Use of phenylpropanoid glycosides in the preparation of ido inhibitors
A technology of phenylpropanoid glycosides and inhibitors, applied to medical preparations containing active ingredients, pharmaceutical formulas, allergic diseases, etc., to achieve a significant inhibitory effect on activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
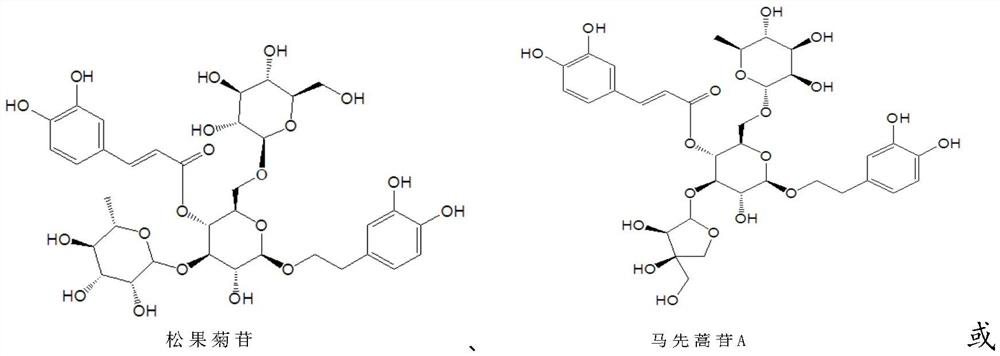
[0034] Example 1 Preparation of echinacoside
[0035] Get the dry fleshy stem of Cistanche deserticola (Cistanche deserticola Ma), add 10 times the volume concentration of the weight and be 75% ethanol aqueous solution and heat and reflux to extract twice, extract for the first time for 2 hours, extract for the second time for 1 hour, combine the extracts, reduce Concentrate under pressure until there is no alcohol smell, to obtain Cistanche deserticola extract;
[0036]Suspend the extract of Cistanche deserticola in 1 times the weight of water, then use n-butanol as the extractant to extract twice, collect the organic phase of the extract, and then concentrate under reduced pressure to obtain the extract of Cistanche deserticola n-butanol extraction part ;
[0037] Purify the extract from the n-butanol extraction part of Cistanche deserticola through AB-8 macroporous resin column chromatography (the diameter of the macroporous resin column is 8cm, and the column volume is 3...
Embodiment 2
[0040] Example 2 Preparation of equine artemisinin A and equine artemisinin H
[0041] Take Artemisia spicata (Pedicularis spicata), add 9 times the weight of ethanol aqueous solution with a volume concentration of 95%, heat and reflux for extraction twice, the first extraction is 2 hours, the second extraction is 1 hour, the combined extracts are concentrated under reduced pressure to nothing. Alcoholic taste, obtained Artemisia fragrans extract;
[0042] Suspend the extract of Artemisia fringa in 1 times the weight of water, then use n-butanol as the extractant to extract twice, collect the organic phase of the extract, and then concentrate it under reduced pressure to obtain the extract of Artemisia fringae n-butanol extraction part ;
[0043] Purify the extract from the n-butanol extraction part of Artemisia fringae by AB-8 macroporous resin column chromatography (the diameter of the macroporous resin column is 8cm, and the column volume is 3.5L), using water as mobile ...
experiment example 1
[0046] Experimental example 1 Study on the inhibitory activity of echinacoside, equine artemisinin A and equine artemisinin H on IDO
[0047] 1. Purpose of the experiment
[0048] HEK293 cells were transfected with the plasmid pcDNA3.1-IDO to highly express IDO, and then the inhibitory activities of echinacoside, equine artemisinin A and equine artemisinin H on IDO at the cellular level were determined respectively.
[0049] 2. Experimental method
[0050] HEK 293 cells were seeded in a 96-well plate at a density of 2.5X104 cells / well, cultured in DMEM medium (containing 10% fetal bovine serum, 50 U / mL penicillin and 50 mg / mL streptomycin), placed at 37 ° C, humidity 95%, 5% CO 2 cultured in an incubator. After culturing for 24 hours, use liposome Lipofectamin 2000 to mediate pcDNA3.1-hIDO plasmid transfection, and divide them into positive control group and experimental group 1-3. The positive control group uses 1-methyltryptophan (1-MT) as the test product, and the exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com