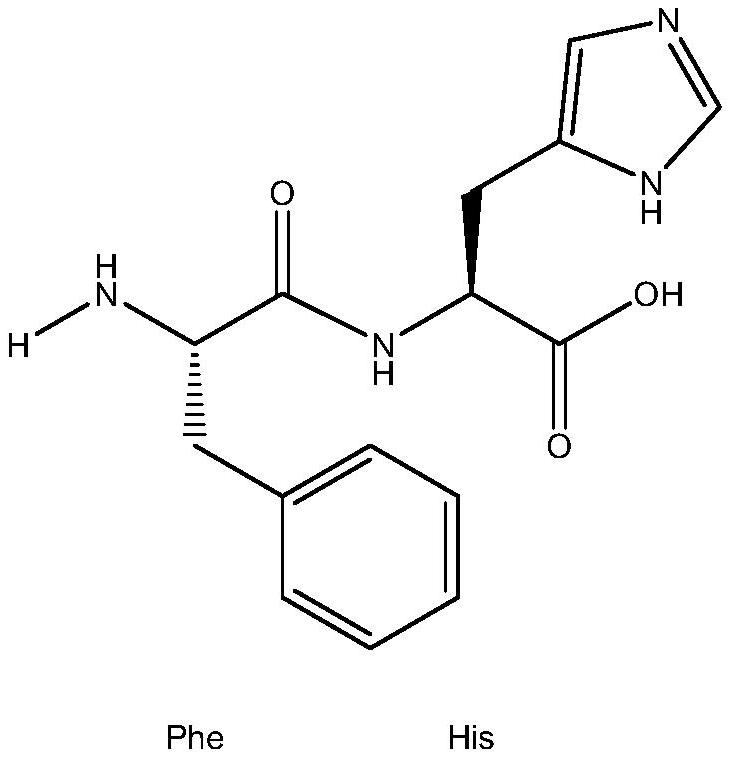
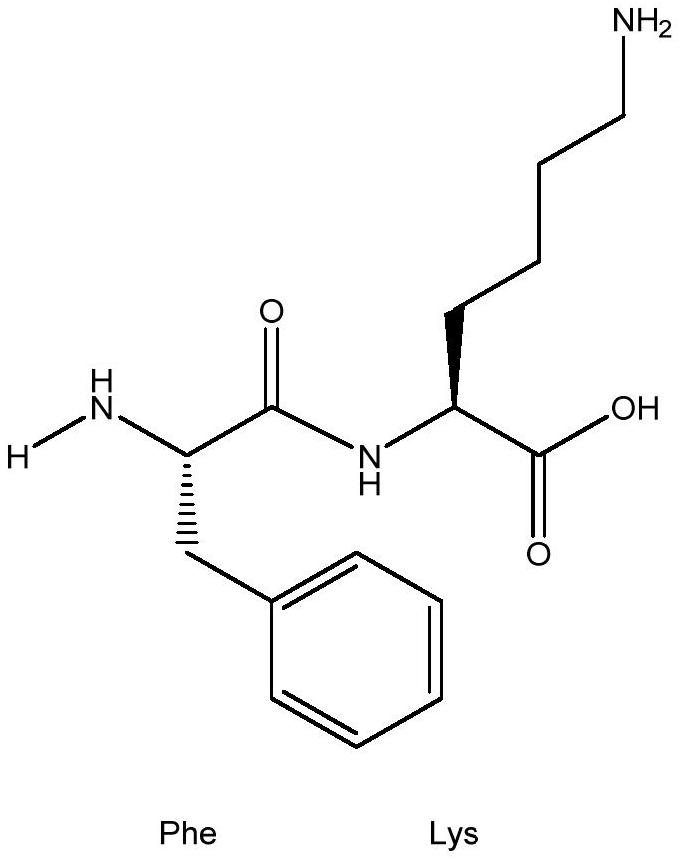
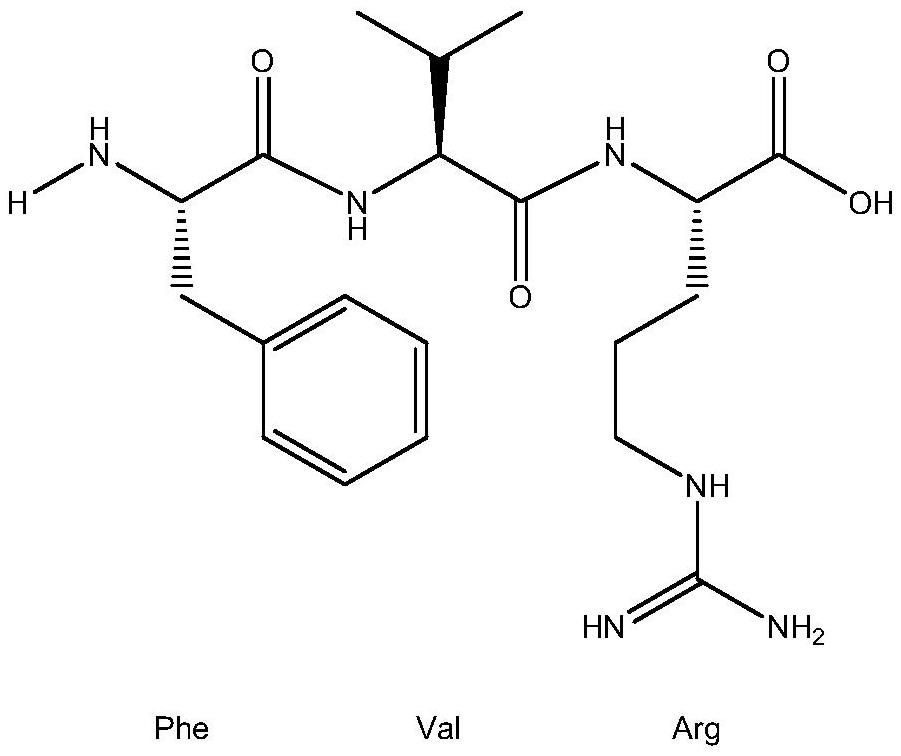
A kind of xanthine oxidase inhibitor containing phenylalanine and its application
A xanthine oxidase and inhibitor technology, applied in the field of functional polypeptides, can solve problems such as reducing uric acid, liver and kidney damage, and achieve a good effect of xanthine oxidase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The method for extracting four kinds of polypeptides FH, FK, FVR and LFW in bonito meat, concrete steps are as follows:
[0030] (1) Protease hydrolysis of bonito meat: After crushing the bonito meat, add water and stir according to the mass ratio of 1:1, heat up to about 55°C, add trypsin with 1.0% of the bonito meat mass, enzymatically hydrolyze for 4 hours, then heat up to 95°C to kill the enzyme After 15 minutes, the enzymatic solution was concentrated by filtration and spray-dried to obtain bonito enzymatic hydrolyzate;
[0031] (2) Ethanol separation: Add 80% (V / V) ethanol to the bonito hydrolyzate at a mass ratio of 1:4, stir at 25°C for 1 hour, centrifuge at 4800×g for 15 minutes, and take the supernatant The alcohol-soluble component of bonito hydrolyzate was obtained after liquid concentration and spray drying;
[0032] (3) Gel column purification: redissolve the alcohol-soluble component of the bonito hydrolyzate with water to a concentration of 20% (W / V), a...
Embodiment 2
[0035] The solid-phase synthesis method of polypeptide FH, the specific steps are as follows:
[0036] Polypeptide FH was synthesized by a solid-phase polypeptide synthesis method, and the Fmoc (9-fluorenylmethoxycarbonyl) group was used as a polypeptide synthesis method for the α-amino protecting group—Fmoc method. First, the Fmoc-protected α-histidine (H) was covalently cross-linked to the resin, and then the protective agent was deprotected by alkaline solution and the carboxyl-activated α-phenylalanine (F) was cross-linked. FH, peptide Segments were finally quantitatively excised from the resin with TFA / dioxymethane. Purification was performed on a C18 reverse phase HPLC preparative column. Finally, the prepared polypeptide sample was rotary evaporated to remove residual organic reagents, etc., and after freeze-drying, it was made into a white powder, which was identified by mass spectrometer, and its purity was analyzed by reverse-phase HPLC. The purity requirement was g...
Embodiment 3
[0040] The solid-phase synthesis method of polypeptide FK, the specific steps are as follows:
[0041] Polypeptide FK is synthesized by a solid-phase polypeptide synthesis method, and the Fmoc (9-fluorenylmethoxycarbonyl) group is used as a polypeptide synthesis method for an α-amino protecting group—Fmoc method. First, the Fmoc-protected α-lysine (K) is covalently cross-linked to the resin, and then the protective agent is deprotected by alkaline solution and the carboxyl-activated α-phenylalanine (F) is cross-linked with FK, and the peptide Finally, it was quantitatively cleaved from the resin with TFA / dioxymethane. Purification was performed on a C18 reverse phase HPLC preparative column. Finally, the prepared polypeptide sample was rotary evaporated to remove residual organic reagents, etc., and after freeze-drying, it was made into a white powder, which was identified by mass spectrometer, and its purity was analyzed by reverse-phase HPLC. The purity requirement was grea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com