Preparation method of quality control sample of nitrofuran metabolite residues in fish muscle
A technology for nitrofuran and quality control samples, which can be used in measuring devices, instruments, scientific instruments, etc., and can solve the problems of long cycle and expensive quality control samples.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
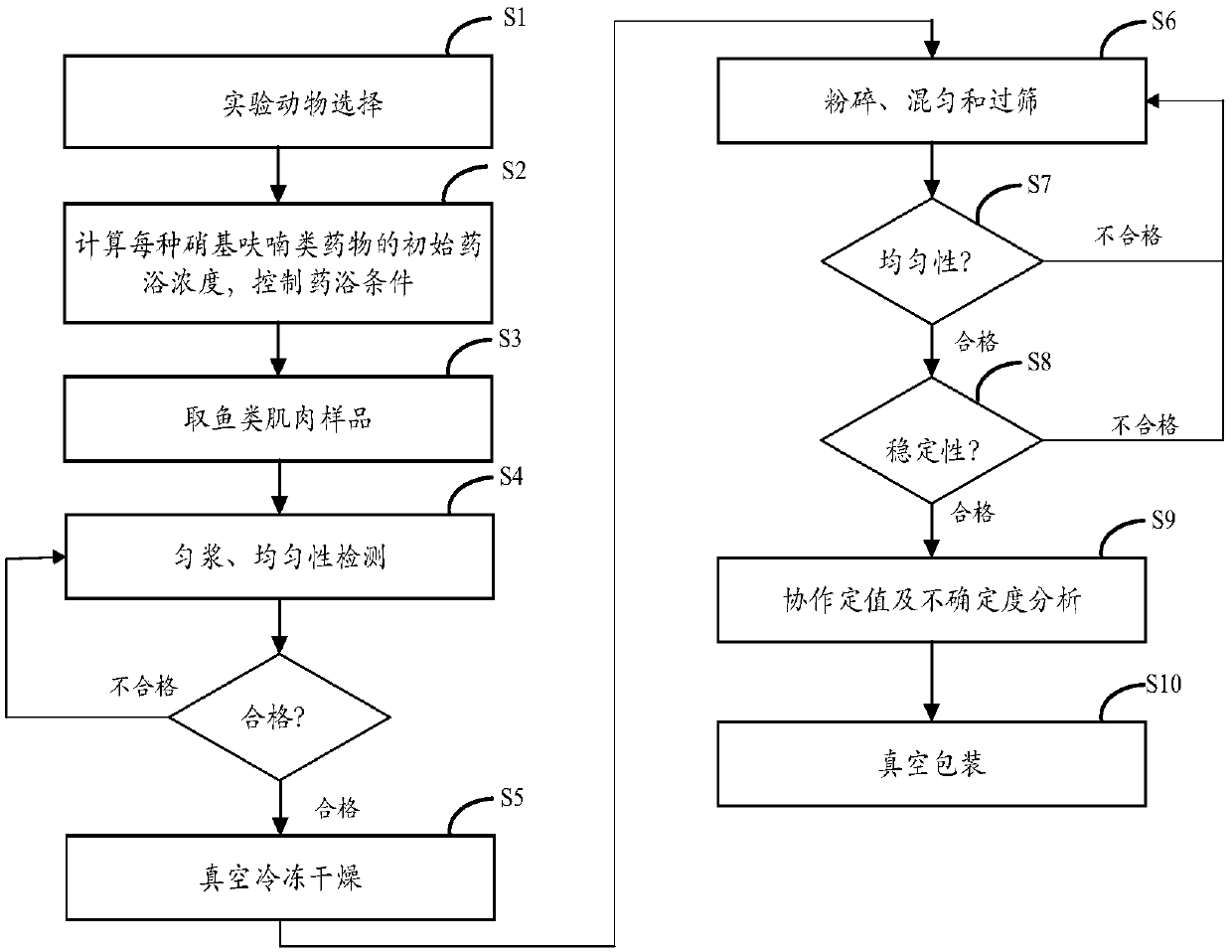
[0046] The preparation method of the residual quality control sample of nitrofuran metabolites in the fish muscle of the present embodiment can comprise the following steps:
[0047] Step S1, selection of experimental animals: select negative live fish that meet the preset size and have not been fed with nitrofuran drugs; optionally, the weight of the negative sample of live fish with the preset size is 900g-1100g / strip.
[0048] Step S2, calculating the initial dipping concentration of each nitrofuran drug, and controlling dipping conditions: after the negative sample adapts to the environment, it is dipped for 10 hours under the set conditions, wherein the dipping pool water body It contains four kinds of nitrofuran drugs: furazolidone, furaltadone, nitrofurazone and nitrofurantoin. Each nitrofuran corresponds to an initial dipping concentration.
[0049] Optionally, calculate the initial bath concentration of each nitrofuran original drug according to the linear equation ...
Embodiment 4
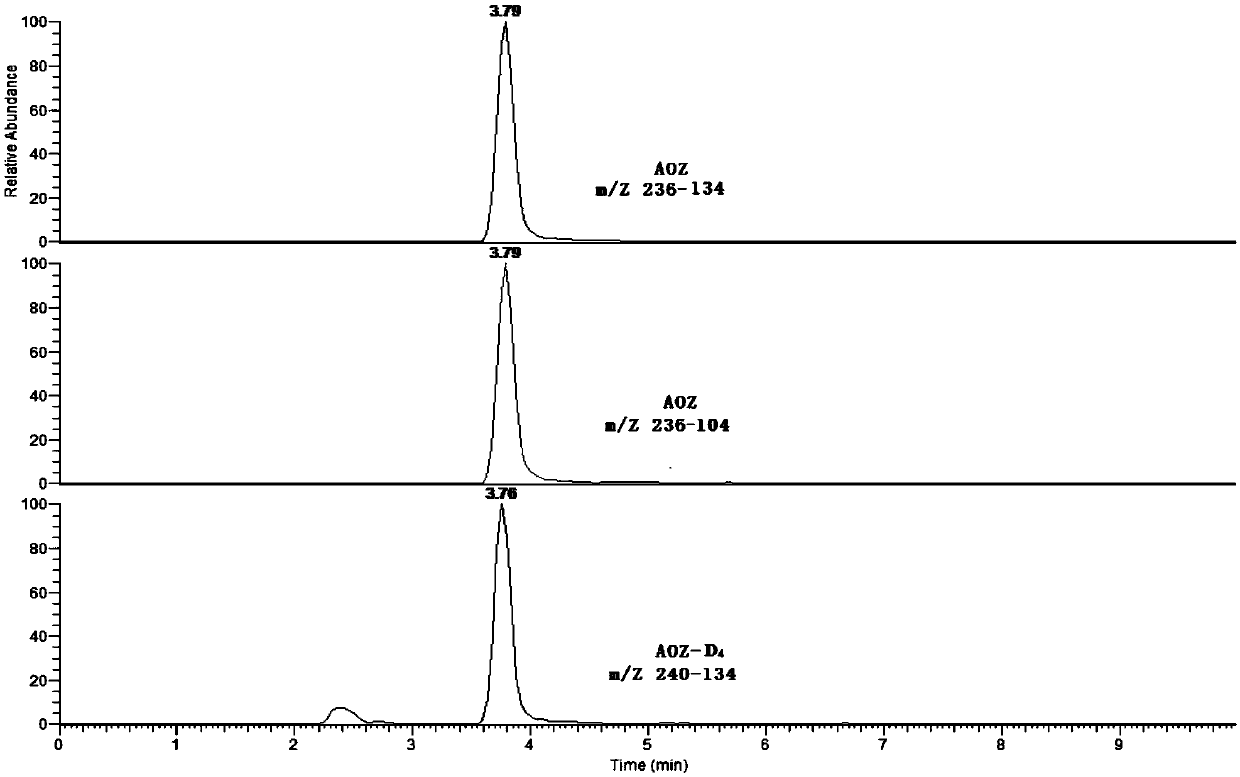
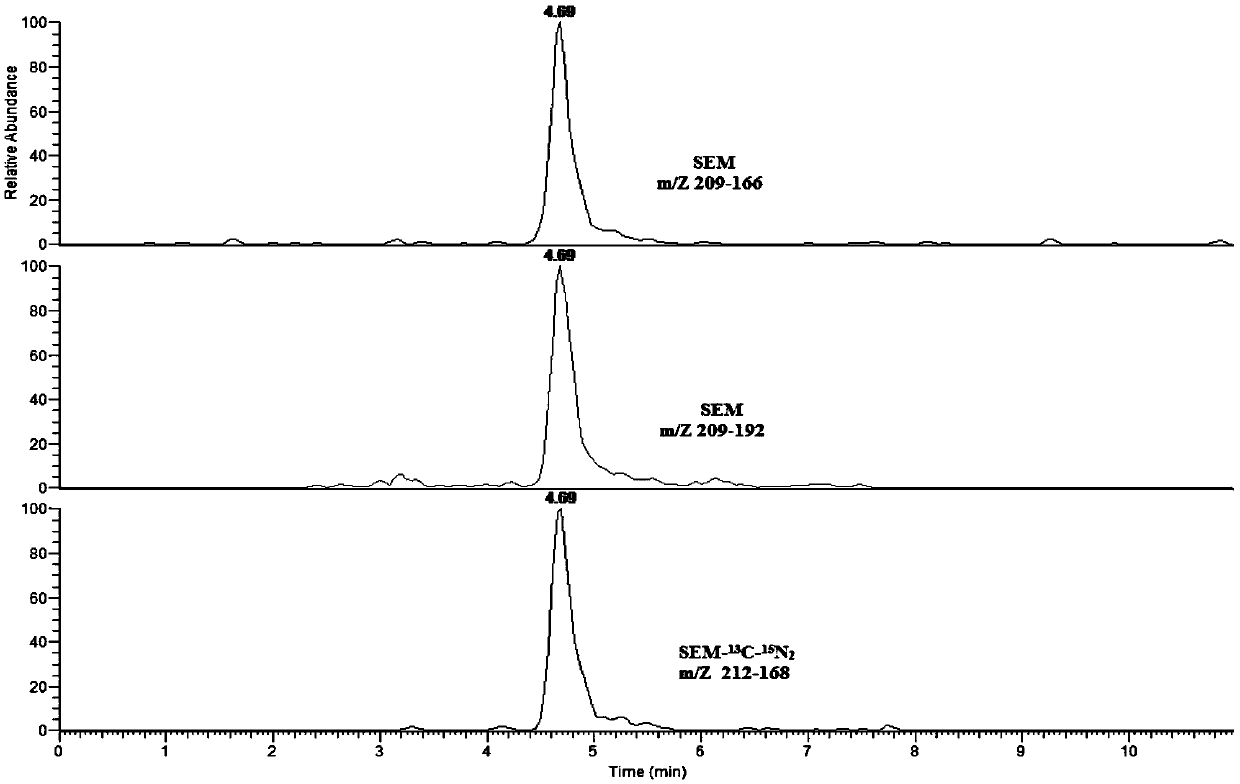
[0141] Valuation and Uncertainty Assessment
[0142] The low-concentration lyophilized powder was detected by liquid chromatography-tandem mass spectrometry by 9 accredited laboratories, and the value was determined collaboratively. The results of the value determination and data processing are shown in Tables 14-17.
[0143] Table 14 SEM measurement results of freeze-dried powder in each laboratory
[0144]
[0145] Table 15 AOZ determination results of freeze-dried powder in each laboratory
[0146]
[0147] Table 16 Determination results of AMOZ of freeze-dried powder in each laboratory
[0148]
[0149] Table 17 AHD measurement results of freeze-dried powder in each laboratory
[0150]
[0151] Firstly, the normality of the distribution of all measured data is examined, and the test results by the Shapiro-Wilk method are as follows:
[0152] (1) SEM Shapiro-Wilk method normal distribution test results for freeze-dried powder samples:
[0153] Table 18 SEM d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com