Eccentric compression dressing for intravenous closure treatment and production method thereof
A production method and post-treatment technology, applied in the direction of drug combinations, pharmaceutical formulas, plant raw materials, etc., can solve problems such as wound infection, achieve the effects of reducing pain, shortening healing time, and promoting the healing of bleeding wounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
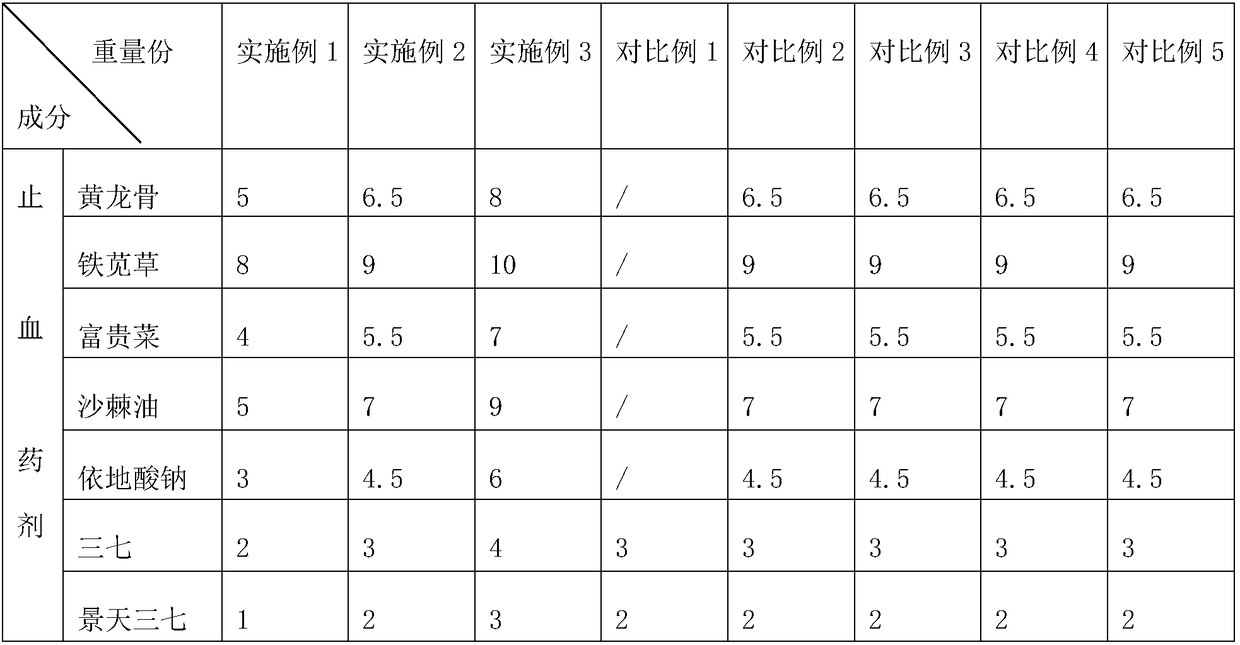
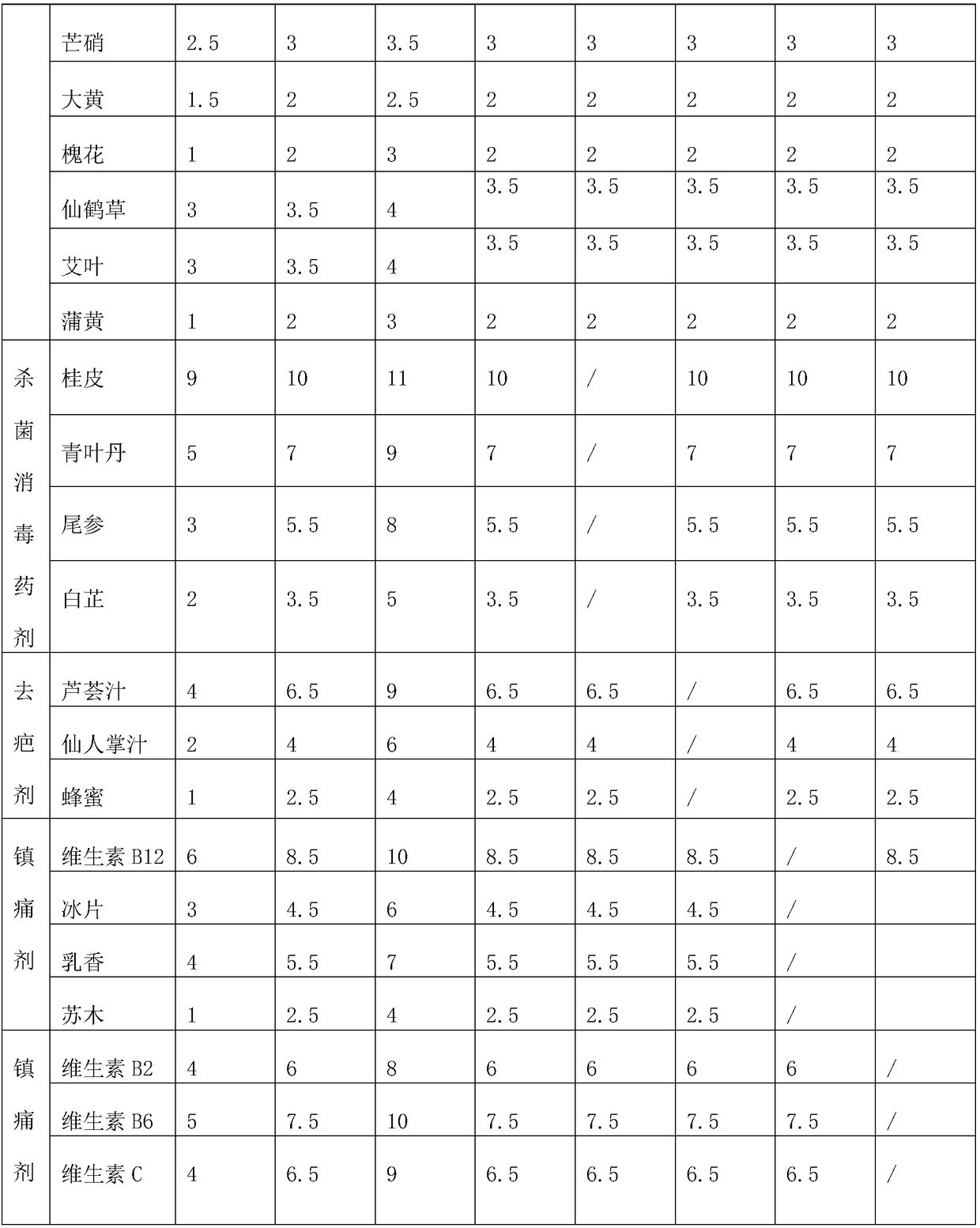
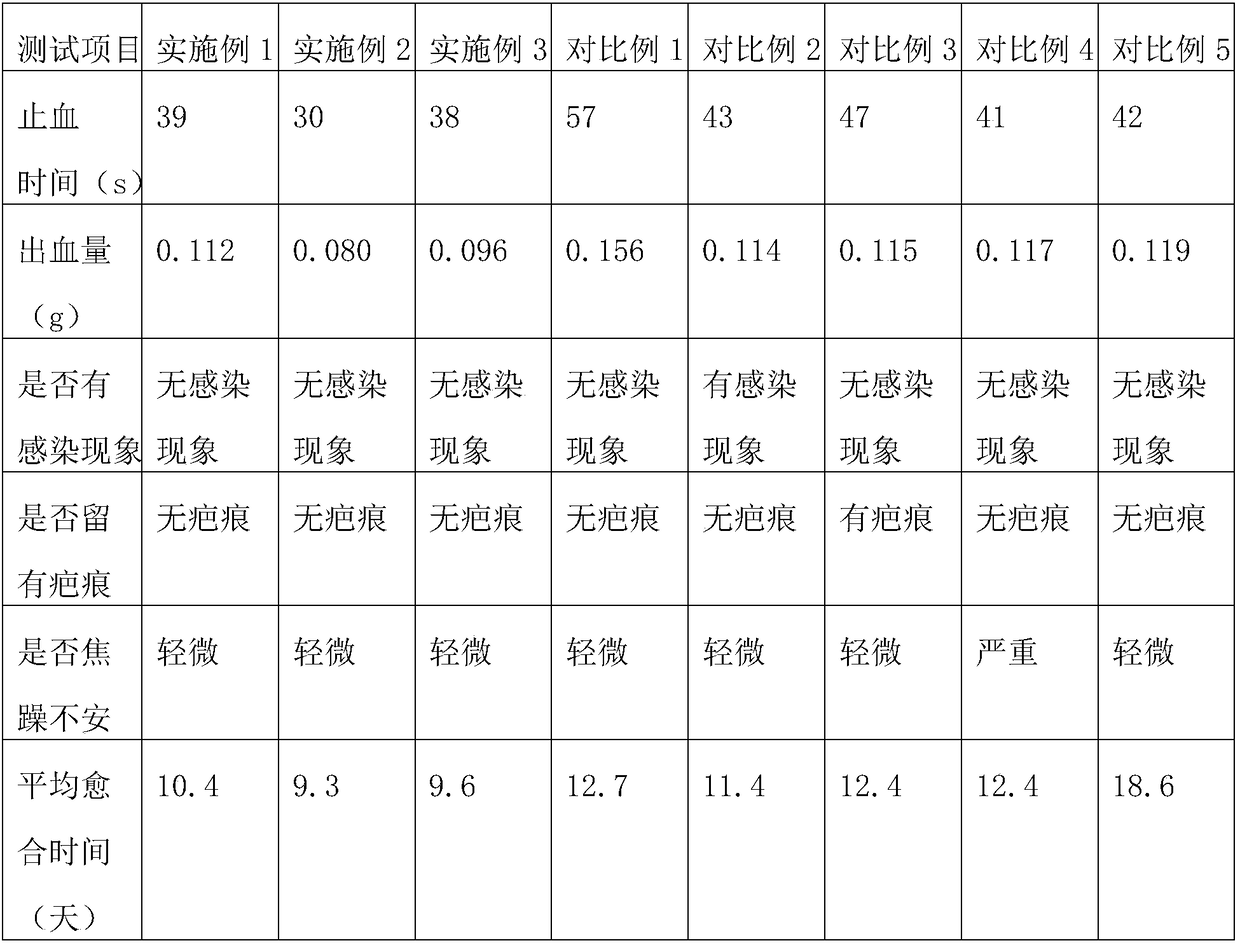
[0043] Embodiment 1: An eccentric compression dressing for venous closure treatment, comprising hemostatic gauze and a mesh cover wrapped in the hemostatic gauze to prevent the hemostatic gauze from spreading; the mesh cover is made of elastic material; the mesh cover and the hemostatic gauze A viscose layer for adhering the hemostatic gauze to the hemostatic gauze is arranged on the contacting side; the viscose layer is made of the following components in parts by mass: 4 parts of nettle root, 5 parts of bletilla striata, 2 parts of dandelion, 3 parts of dandelion 1 part forsythia and 1 part puffball.
[0044] Wherein the hemostatic gauze comprises fabric, the hemostatic agent impregnated on the fabric, bactericidal disinfectant, scar remover, analgesic and healing agent; wherein the mass ratio of hemostatic agent, bactericidal disinfectant, scar remover, analgesic and healing agent is 1.3:0.6:0.2:0.1:0.3;
[0045] The hemostatic agent is prepared from the following ingredie...
Embodiment 2
[0057] Embodiment 2: An eccentric compression dressing for venous closure treatment, comprising hemostatic gauze and a mesh cover wrapped outside the hemostatic gauze to prevent the hemostatic gauze from spreading; the mesh cover is made of elastic material; the mesh cover and hemostatic A viscose layer for adhering the hemostatic gauze to the hemostatic gauze is arranged on the side where the gauze contacts; 3.5 parts forsythia and 2 parts puffball.
[0058] Wherein the hemostatic gauze comprises fabric, the hemostatic agent impregnated on the fabric, bactericidal disinfectant, scar remover, analgesic and healing agent; wherein the mass ratio of hemostatic agent, bactericidal disinfectant, scar remover, analgesic and healing agent is 1.4:0.75:0.3:0.15:0.4;
[0059] The hemostatic agent is prepared from the following ingredients in parts by mass: 6.5 parts of huanglongwei, 9 parts of iron amaranth, 5.5 parts of rich cabbage, 7 parts of seabuckthorn oil, 4.5 parts of sodium ed...
Embodiment 3
[0071] Embodiment 3: An eccentric compression dressing for venous closure treatment, comprising hemostatic gauze and a mesh cover wrapped in the hemostatic gauze to prevent the hemostatic gauze from spreading; the mesh cover is made of elastic material; the mesh cover and the hemostatic gauze A viscose layer for adhering the hemostatic gauze to the hemostatic gauze is arranged on the contacting side; the viscose layer is made of the following components in parts by mass: 6 parts of nettle root, 8 parts of bletilla striata, 3 parts of dandelion, 4 parts by mass. 1 part forsythia and 3 parts puffball.
[0072] Wherein the hemostatic gauze comprises fabric, the hemostatic agent impregnated on the fabric, bactericidal disinfectant, scar remover, analgesic and healing agent; wherein the mass ratio of hemostatic agent, bactericidal disinfectant, scar remover, analgesic and healing agent is 1.53:0.9:0.4:0.2:0.5;
[0073] The hemostatic agent is prepared from the following ingredient...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap