A kind of preparation method and application of 2-hydroxymethyl oxetane derivative
A technology of trimethylsilyl and phenyl, applied in the field of preparation of 2-hydroxymethyl oxetane derivatives, can solve problems such as being unsuitable for industrial production, uneconomical and environmentally friendly, and expensive catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
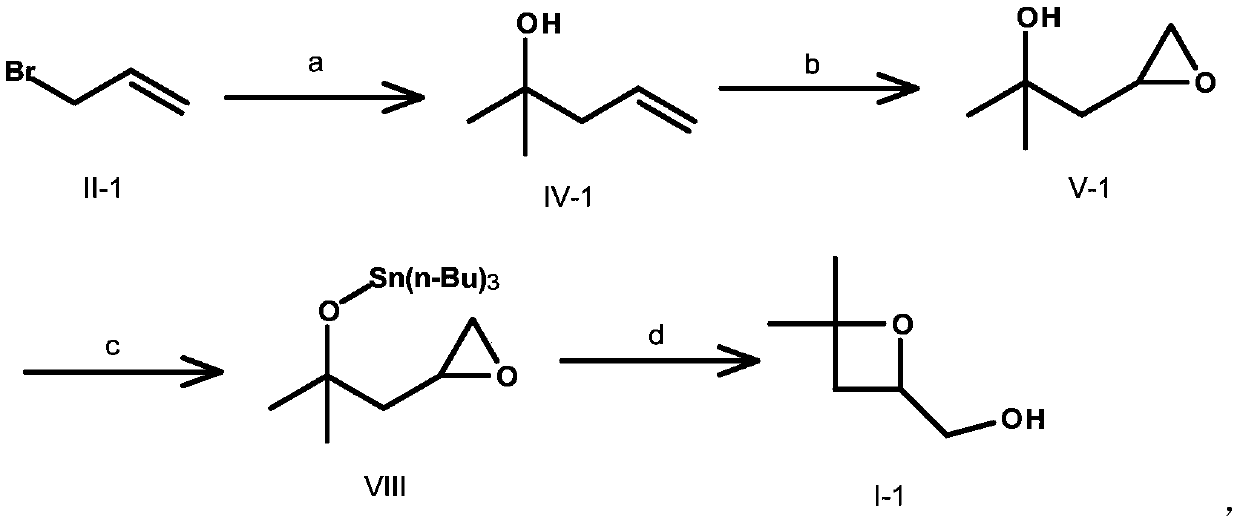
[0041] Preparation of Compound IV-1
[0042] Compound II-1 (400g, 3.30mol, 1.0e.q.), compound III-1 (480g, 8.26mol, 2.5e.q.) were dissolved in 1.5L ammonium chloride (320g) aqueous solution, and Zn powder (216.2g, 3.30mol, 1.0e.q.), control T1 H-NMR (400MHz, CDCl 3 ) δ (ppm) 1.24 (s, 6H), 2.24-2.26 (d, 2H), 5.11-5.18 (m, 2H), 5.86-5.93 (m, 1H).
[0043] Preparation of Compound V-1
[0044] Dissolve compound IV-1 (160.0g, 1.59mol, 1.0e.q.) in 2L of DCM, add m-CPBA (389.8g, 1.92mol, 1.2e.q.) in batches, exotherm, control T1 H-NMR (400MHz, CDCl3) δ (ppm) 1.33-1.35 (d, 6H), 1.55-1.60 (q, 1H), 1.81-1.86 (dd, 1H), 1.94 (s, 1H), 2.48-2.50 ( m, 1H), 2.80-2.82(t, 1H), 3.11-3.16(m, 1H).
[0045] Preparation of compound VI-1
[0046] NaH (31.43g, 0.78mol, 1.1e.q.) was dissolved in 400mL of THF, benzyl alcohol (100mL) was added dropwise under an ice-water bath, and after stirring for 30min, compound V-1 (83.0g, 0.71mol, 1.0e.q. ), after the dropwise addition, stirred at r...
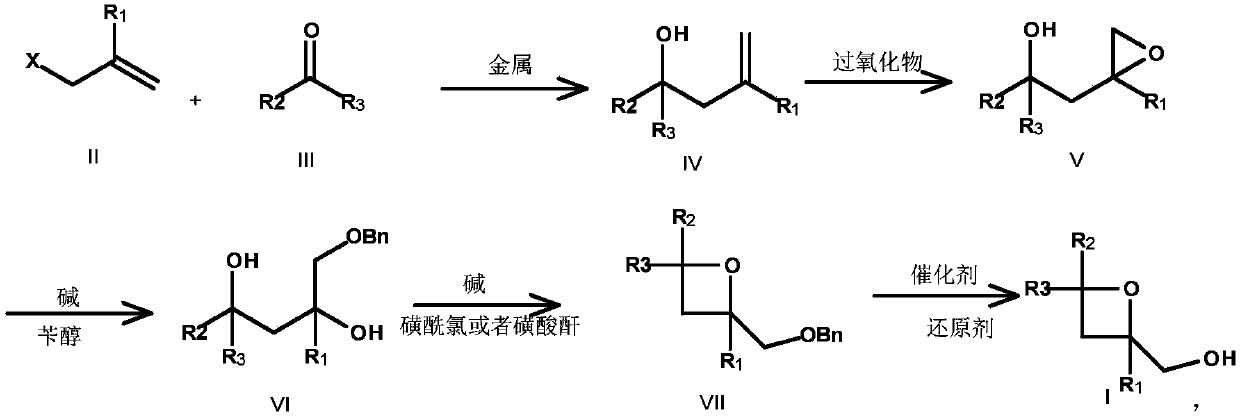
Embodiment 2
[0052]
[0053] Preparation of Compound IV-2
[0054] Compound II-2 (100g, 0.74mol, 1.0e.q.), compound III-1 (133.5g, 1.11mol, 1.5e.q.) were dissolved in 1.0L aqueous solution, and Cu powder (94.04g, 1.48mol, 2.0e.q. ), control T<50°C, after the addition is complete, stir at room temperature for 3 h, filter, wash the filter cake with MTBE, extract the aqueous phase with MTBE, combine the organic phases, wash with 1L saturated brine, dry and concentrate to obtain compound IV-2 as 71.73 g of light yellow liquid, yield 55%.
[0055] Preparation of Compound V-2
[0056] Compound IV-2 (70g, 0.39mol, 1.0e.q.) was dissolved in 800mL of 1,4-dioxane, and tert-butanol peroxide (70.29g, 0.78mol, 2.0e.q.) was added in portions at -10°C , after the addition is complete, stir and react at room temperature for 16 hours, add 1L sodium thiosulfate (80g) aqueous solution, stir and react for 1 hour, filter and separate the liquids, wash the organic phase with saturated brine, dry over anhyd...
Embodiment 3
[0064]
[0065] Preparation of Compound IV-3
[0066] Compound II-3 (100g, 0.507mol, 1.0e.q.), compound III-3 (106.7g, 1.52mol, 3.0e.q.) were dissolved in 800L sodium acetate (80g) aqueous solution, and Mg powder (36.96g, 1.521 mol, 3.0e.q.), control T<50°C, after the addition, T=40°C and stir for 8h, filter, wash the filter cake with MTBE, extract the aqueous phase with MTBE, combine the organic phases, wash with 1L saturated brine, and dry Concentration gave 60.41 g of compound IV-3 as a light yellow liquid, with a yield of 63.3%. Preparation of Compound V-3
[0067] Dissolve IV-3 (60.0g, 0.318mol, 1.0e.q.) in 600mL of DCM solution, add carbamide peroxide (87.81g, 0.956mol, 3.0e.q.) in batches at 0°C, exotherm, control T<40°C , after the addition was completed, stirred and reacted at T=10°C for 16 h, added 1 L of sodium thiosulfate (100 g) aqueous solution, stirred and reacted for 1 h, filtered and separated, and the organic phase was washed with saturated brine, dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com