A kind of lead halide hybrid material and preparation method thereof
A lead chloride and lead bromide technology, applied in the field of fluorescent emitters, can solve the problems of poor luminous stability, instability, and reduced luminous intensity, and achieve high luminous performance and high stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Weigh 0.11g of lead chloride and 0.0344g of trans-1,4-cyclohexanedicarboxylic acid into a 15ml autoclave, add 10ml of a mixed solvent of N,N-dimethylformamide and ethanol, and then add 0.2ml of perchloric acid, followed by ultrasonication for half an hour to make it evenly mixed, then put it into an oven, heat up to 120°C at a rate of 15°C / hour, and react for 72 hours. After the reaction, cool down the system at a rate of 15°C / hour Speed to 20°C, transfer the flaky colorless crystals to a beaker, wash with ethanol three times, and then dry in a constant temperature oven at 60°C.
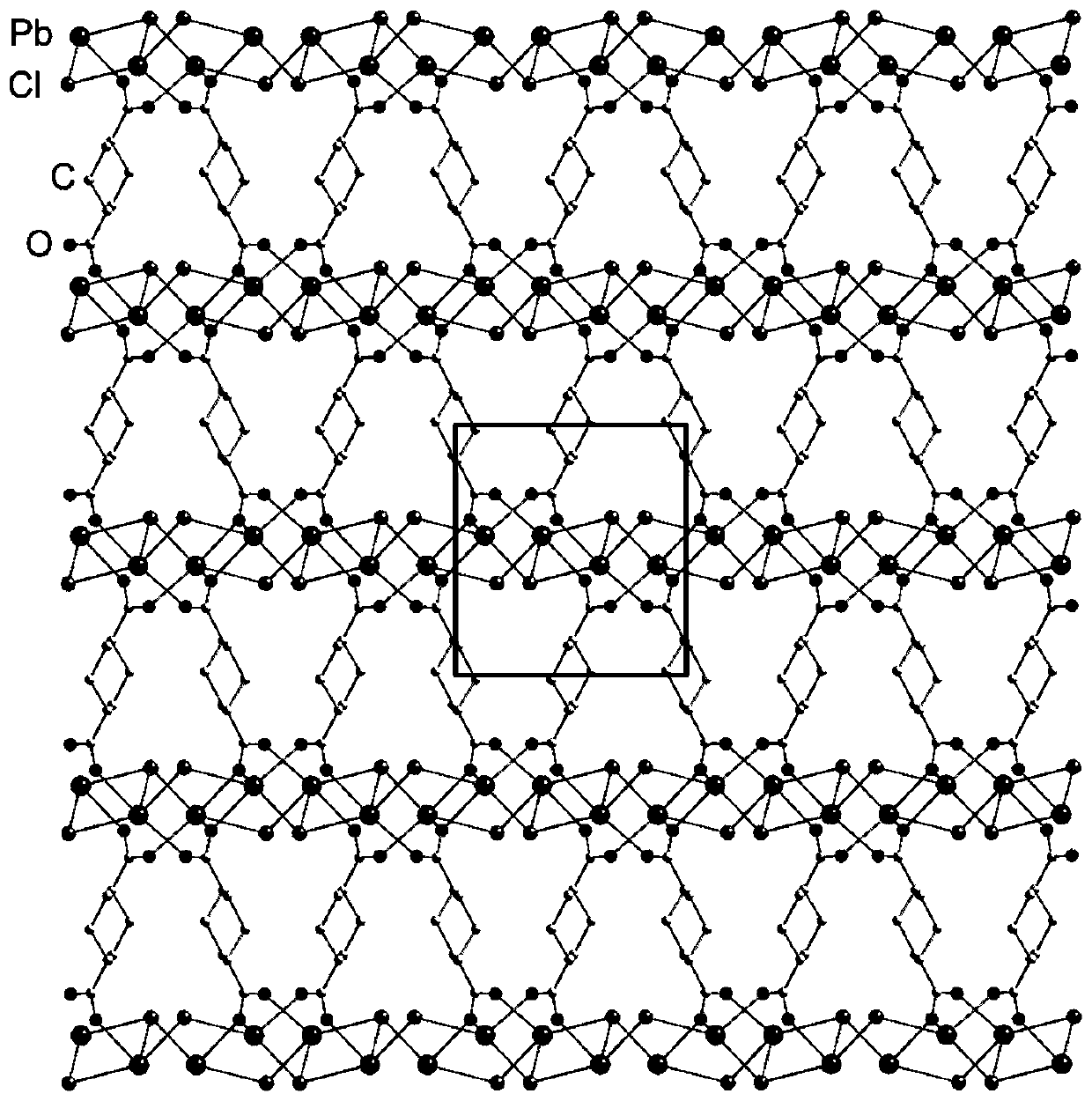
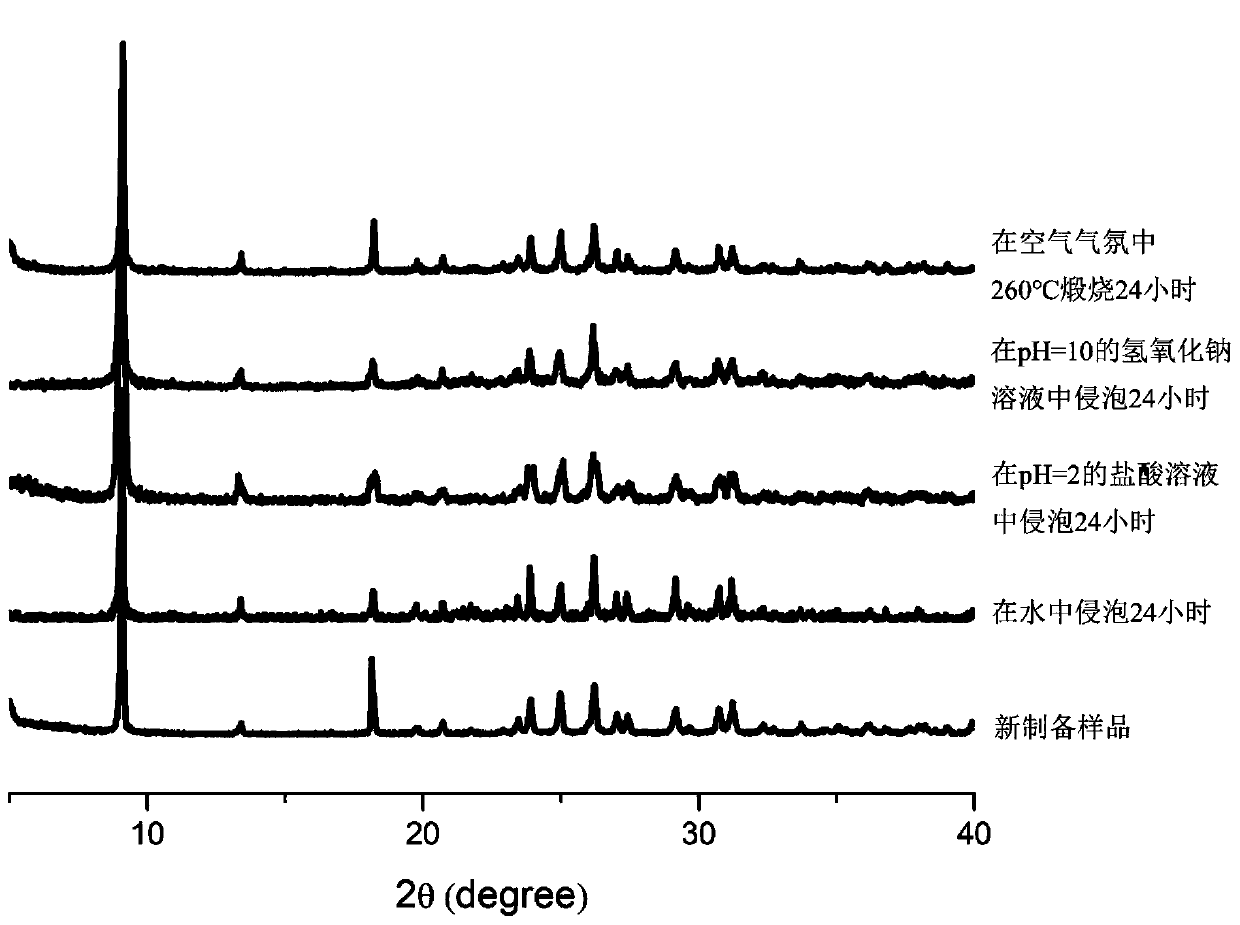
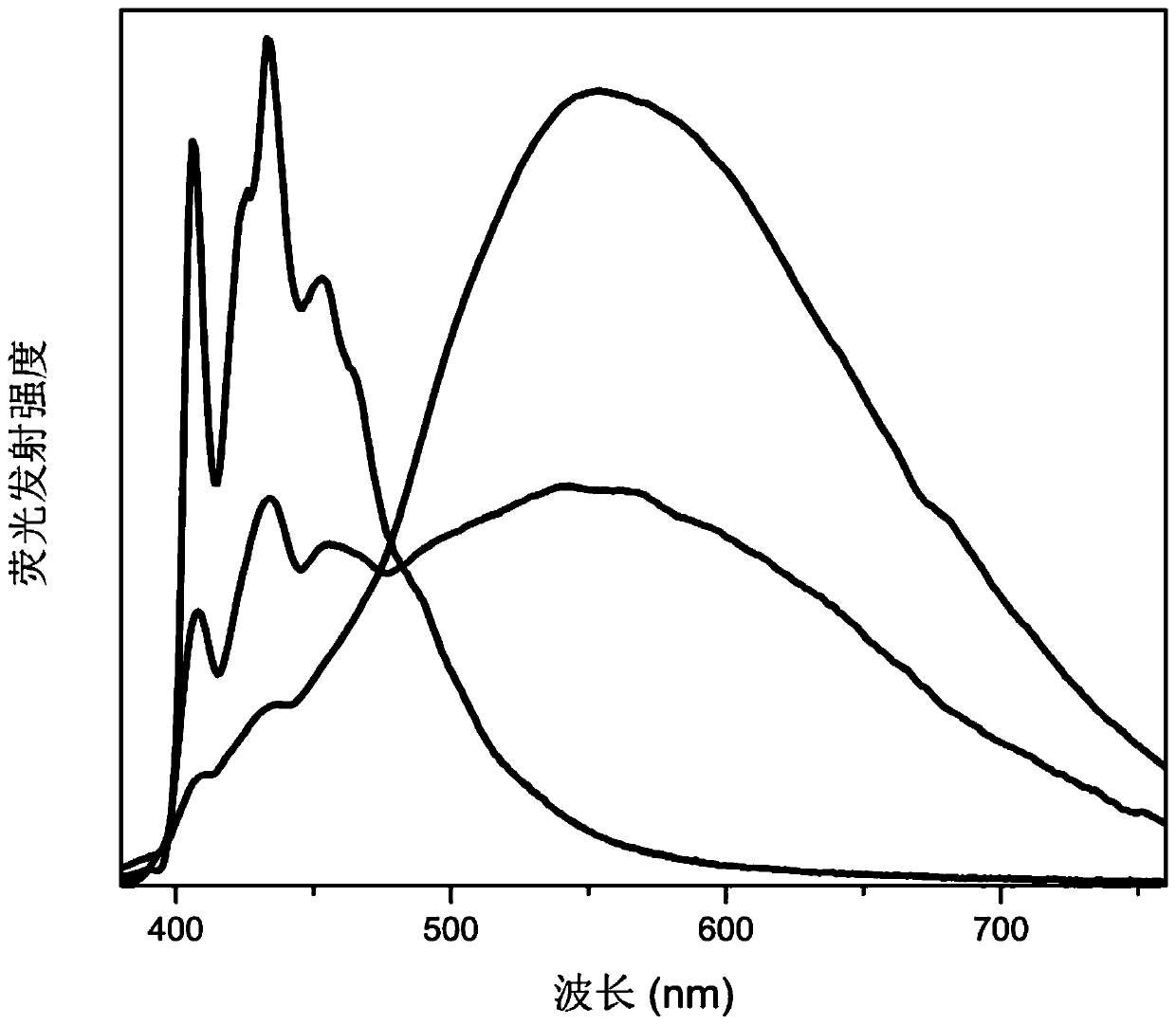
[0041] The properties of the material obtained in this embodiment are no flaky crystals. Confirmed by single crystal X-ray diffraction characterization, the frame connection structure of the material is as follows: figure 1 As shown, it can be seen that the material possesses a highly distorted two-dimensional lead chloride inorganic layer.
[0042] Material [Pb 2 Cl 2 2+ ][ - o 2 C(C...
Embodiment 2
[0047] Weigh 0.278g of lead chloride and 0.172g of trans-1,4-cyclohexanedicarboxylic acid into a 50ml autoclave, add 20ml of a mixed solvent of N,N-dimethylformamide and ethanol, and then add 200μl perchloric acid, followed by ultrasound for half an hour to make it evenly mixed, then put it in an oven, raise the temperature to 120°C, and react for 72 hours. The flaky colorless crystals were transferred to a beaker, washed three times with ethanol, and then dried in a constant temperature oven at 60°C. The properties of the material obtained in this example are colorless flaky crystals.
Embodiment 3
[0049] Weigh 0.278g of lead chloride and 0.344g of trans-1,4-cyclohexanedicarboxylic acid into a 50ml autoclave, add 30ml of a mixed solvent of N,N-dimethylformamide and ethanol, and then add 200μl perchloric acid, followed by ultrasound for half an hour to make it evenly mixed, then put it in an oven, raise the temperature to 120°C, and react for 72 hours. The flaky colorless crystals were transferred to a beaker, washed three times with ethanol, and then dried in a constant temperature oven at 60°C. The properties of the material obtained in this example are colorless flaky crystals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| color rendering index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com