Histamine dihydrochloride combinations and uses thereof
A technology of histamine receptors and subjects, which is applied in the directions of drug combinations, active ingredients of iodine compounds, and medical preparations containing active ingredients, etc., can solve the problems of cancer treatment not being the best effective, ineffective tumor cell killing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0164] Combination of anti-PD-1 / anti-PDL1 and histamine - method
[0165] The EL-4 thymoma cell line (provided by Dr.Ingo Schmitz, Otto-von-Guericke-University, Magdeburg, Germany) was cultivated in DMEM medium (Sigma, Stockholm, Switzerland) supplemented with 10 % fetal bovine serum, 100 U / ml penicillin, 100 μg / ml streptomycin and 2 mM L-glutamine, as in Martner, A. et al. The Journal of Immunology, Vol. 194, No. 10, pp. 5014-5021 mentioned. C57BL / 6J mice (6-8 weeks old, purchased from Charles River Laboratories, Sulzfeld, Germany) were treated with 1.75-2x10 5 EL-4 tumor cells were inoculated subcutaneously and started on the day before cell inoculation by intraperitoneal injection three times a week with saline (control) or 1.5 mg histamine dihydrochloride diluted in saline (purchased from Sigma-Aldrich, Stockholm , Germany) for processing. Three, six and ten days after tumor inoculation, two hundred and forty μg of an antibody against the programmed cell death-1 recep...
example 2
[0167] Combination of anti-PD-1 / anti-PDL1 and histamine - results
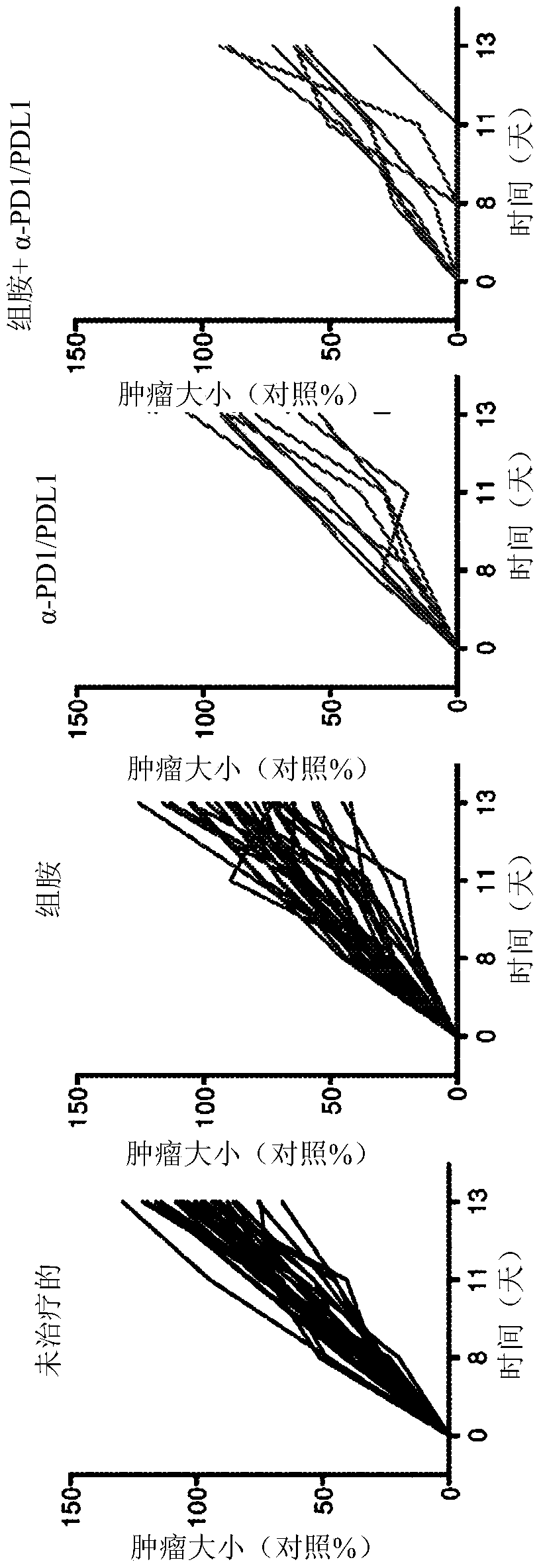
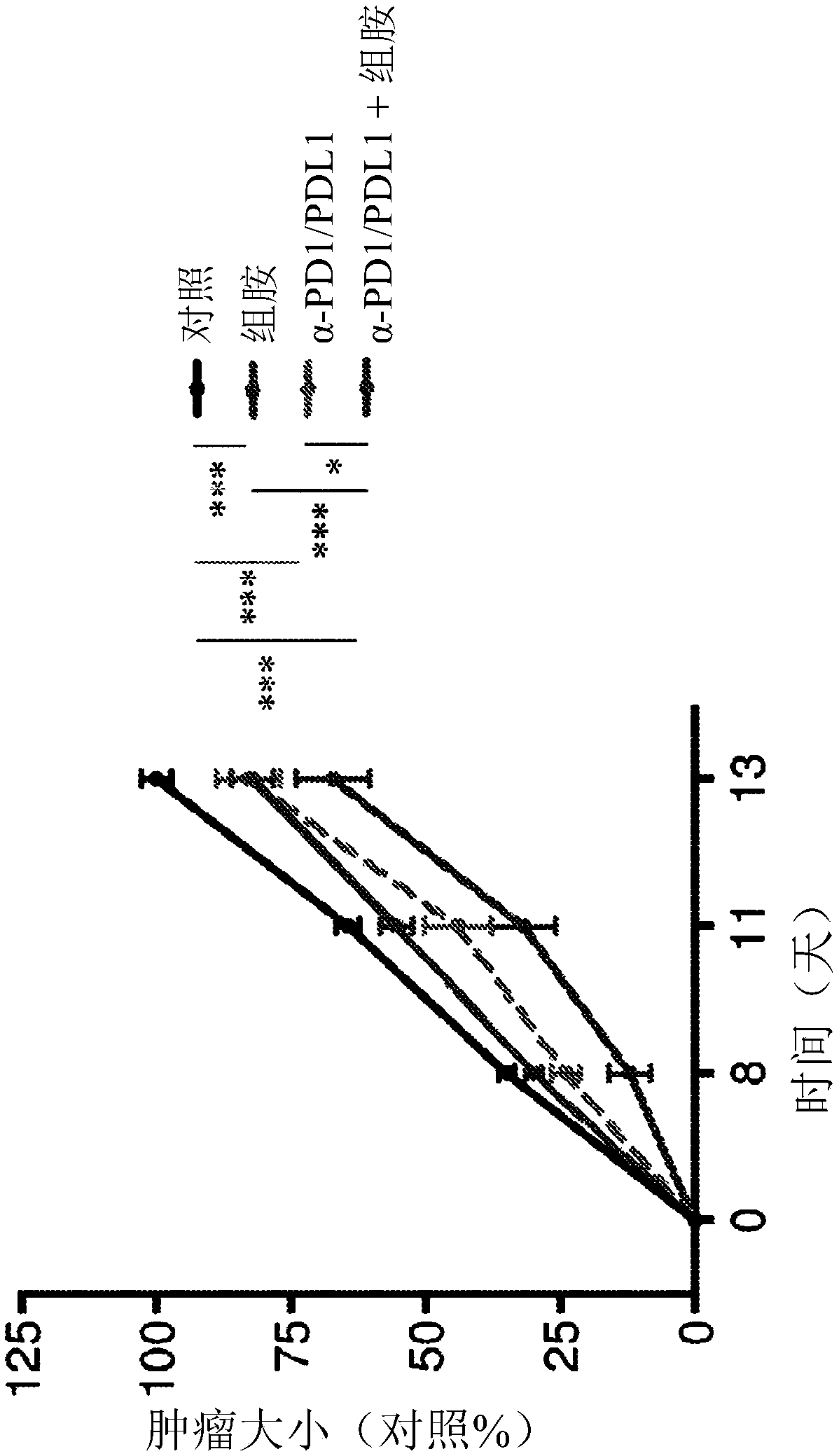
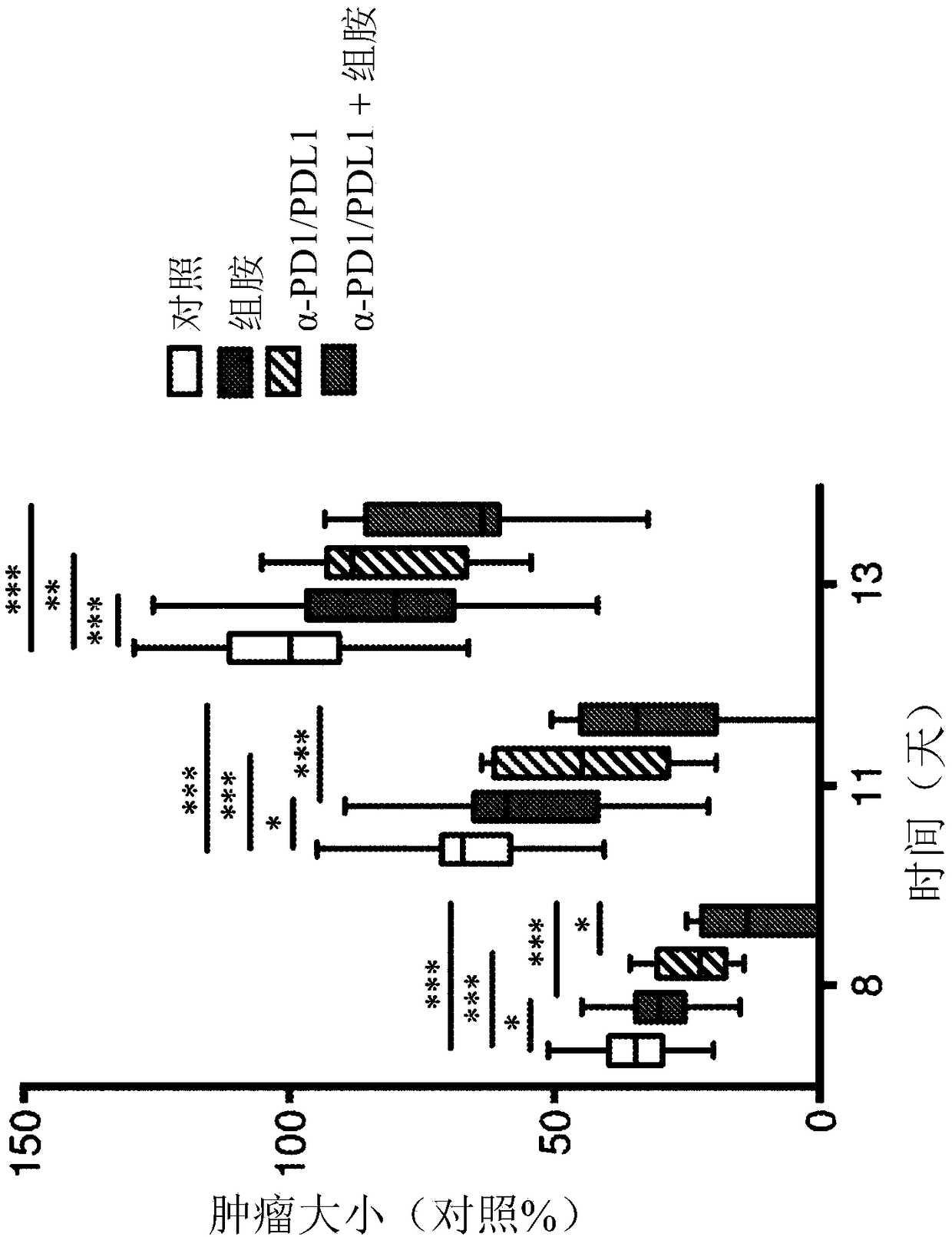
[0168] Consistent with previous studies (Martner, A. et al. The Journal of Immunology, Vol. 194, No. 10, pp. 5014-5021), treatment of EL-4-bearing mice with histamine significantly reduced tumor growth rate ( Figure 1-3 ). Administration of α-PD1-+α-PDL1 inhibitory antibodies resulted in a comparable reduction in tumor growth. The combination of histamine and anti-PD1 / anti-PDL1 increased the reduction in EL-4 tumor growth over each treatment alone (histamine or α-PD1 / α-PDL1). exist Figure 1-3 In , tumor size is indicated as % of control, where 100% is the mean size of control tumors at the end of the experiment (ie, 2 weeks after tumor inoculation).
example 3
[0170] Dynamics of cytotoxic T cell subsets during immunotherapy predict outcome in acute myeloid leukemia
[0171] Materials and Methods
[0172] Patients, Study Design and Objectives
[0173] This single-arm, multicenter Phase IV study (Relapse:Remission, NCT01347996, registered at www.clinicaltrials.gov) enrolled 84 patients (ages 18-79) with AML in first CR. Such as Figure 4 As shown schematically in , patients received ten consecutive 21-day cycles of histamine dihydrochloride (HDC; Ceplene) in combination with low-dose IL-2 over an 18-month period or until relapse or death.
[0174] Treatment continued for a total of 18 months or until the patient relapsed, died, discontinued treatment due to an adverse event, withdrew consent, or became lost to follow-up. Cycles 1 to 3 consisted of 3 weeks on treatment and 3 weeks off treatment, and in cycles 4 to 10 the off treatment period was extended to 6 weeks. During each cycle, patients in the treatment arm received 0.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com