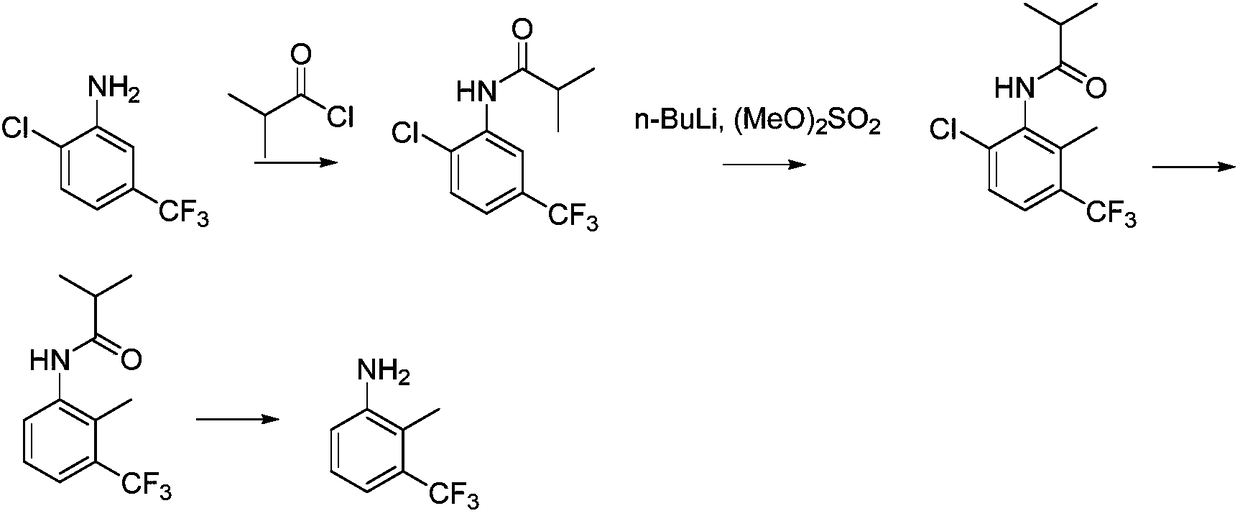
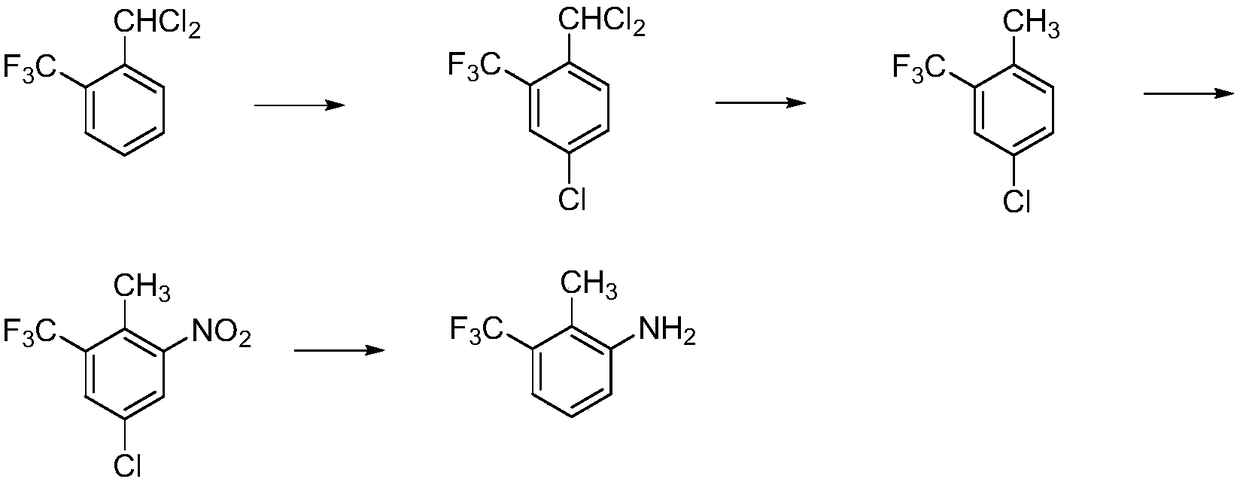
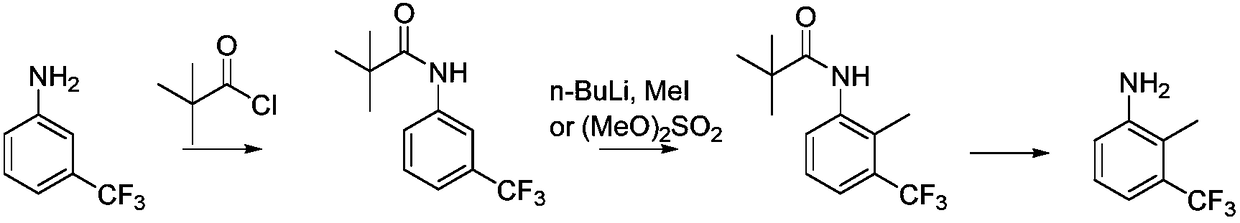Synthetic method for 2-methyl-3-trifluoromethylaniline
A technology of trifluoromethylaniline and a synthesis method, which is applied in the preparation of amino compounds, chemical instruments and methods, preparation of amino compounds from amines, etc., can solve the problems of cumbersome experimental process and poor operability, and achieves easy handling and environmental protection pressure. Small, production-safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1, Synthesis of 6-chloro-2-(methylthio)methyl-3-trifluoromethylaniline:
[0039] Weigh 606.2g of 2-chloro-3-trifluoromethylaniline, add 4L of dichloroethane, stir evenly, add 280.2g of dimethyl sulfide, N-chlorosuccinimide to the system, and control the adding speed , so that the temperature of the system is not higher than 30 degrees, after the addition, the system is stirred at room temperature for 6 hours, then 636g of triethylamine is added to the system, the system is heated to reflux, the temperature is lowered, water is added, liquid separation, desolventization, and the residue is reduced Pressure distillation, the product 635.8g yield 80%, HPLC purity greater than 97%. 1 H NMR (600MHz, CDCl 3 )δ7.304(d, J=8.4Hz, 1H), 7.016(d, J=8.4Hz, 1H), 5.2–4.7(br s, 2H), 3.885(s, 2H), 2.051(s, 3H)
[0040] 2. Synthesis of 6-chloro-2-chloromethyl-3-trifluoromethylaniline hydrochloride:
[0041] Weigh 568.2g of 6-chloro-2-(methylthio)methyl-3-trifluoromethylaniline in a re...
Embodiment 2
[0045] 1, Synthesis of 6-chloro-2-(methylthio)methyl-3-trifluoromethylaniline:
[0046] Weigh 1170g of 2-chloro-3-trifluoromethylaniline, add 17L of dichloroethane, stir evenly, add 354g of dimethyl sulfide and 961g of N-chlorosuccinimide to the system, control the adding speed, Make the temperature of the system not higher than 30 degrees. After the addition, the system is stirred at room temperature for 6 hours, then 1214g of triethylamine is added to the system, the system is heated to reflux, the temperature is lowered, water is added, the liquid is separated, the solvent is removed, and the residue is decompressed. Distilled to obtain the product 1193g yield 78%, HPLC purity greater than 95%. 1 H NMR (600MHz, CDCl 3 )δ7.304(d, J=8.4Hz, 1H), 7.016(d, J=8.4Hz, 1H), 5.2–4.7(br s, 2H), 3.885(s, 2H), 2.051(s, 3H)
[0047] 2. Synthesis of 6-chloro-2-chloromethyl-3-trifluoromethylaniline hydrochloride:
[0048] Weigh 1020g of 6-chloro-2-(methylthio)methyl-3-trifluoromethylani...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com