A medicine composition for treating renal interstitial fibrosis
A kind of technology of renal interstitial fibrosis and composition, which can be applied in the field of medicine and can solve the problems of rosiglitazone and puerarin in renal interstitial fibrosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Cell culture
[0029] HK-2 cells (human renal tubular epithelial cells) were cultured in DMEM / F12 medium containing 10% FBS (fetal bovine serum) and 1% double antibody, and cultured in a cell culture box until the cells grew to 70% When -80% confluent, digest with 0.05% trypsin-EDTA solution. For the experiment, the above-mentioned HK-2 cells were placed in low-sugar DMEM medium for 2 passages, seeded in a 96-well plate at 5000 cells / well, and grown adherently in a cell culture incubator for 24 hours, then replaced with serum-free Wushuang Antibiotics were cultured overnight in low-glucose DMEM medium. Afterwards, the old culture medium was discarded and processed in groups.
[0030] 2. Grouping
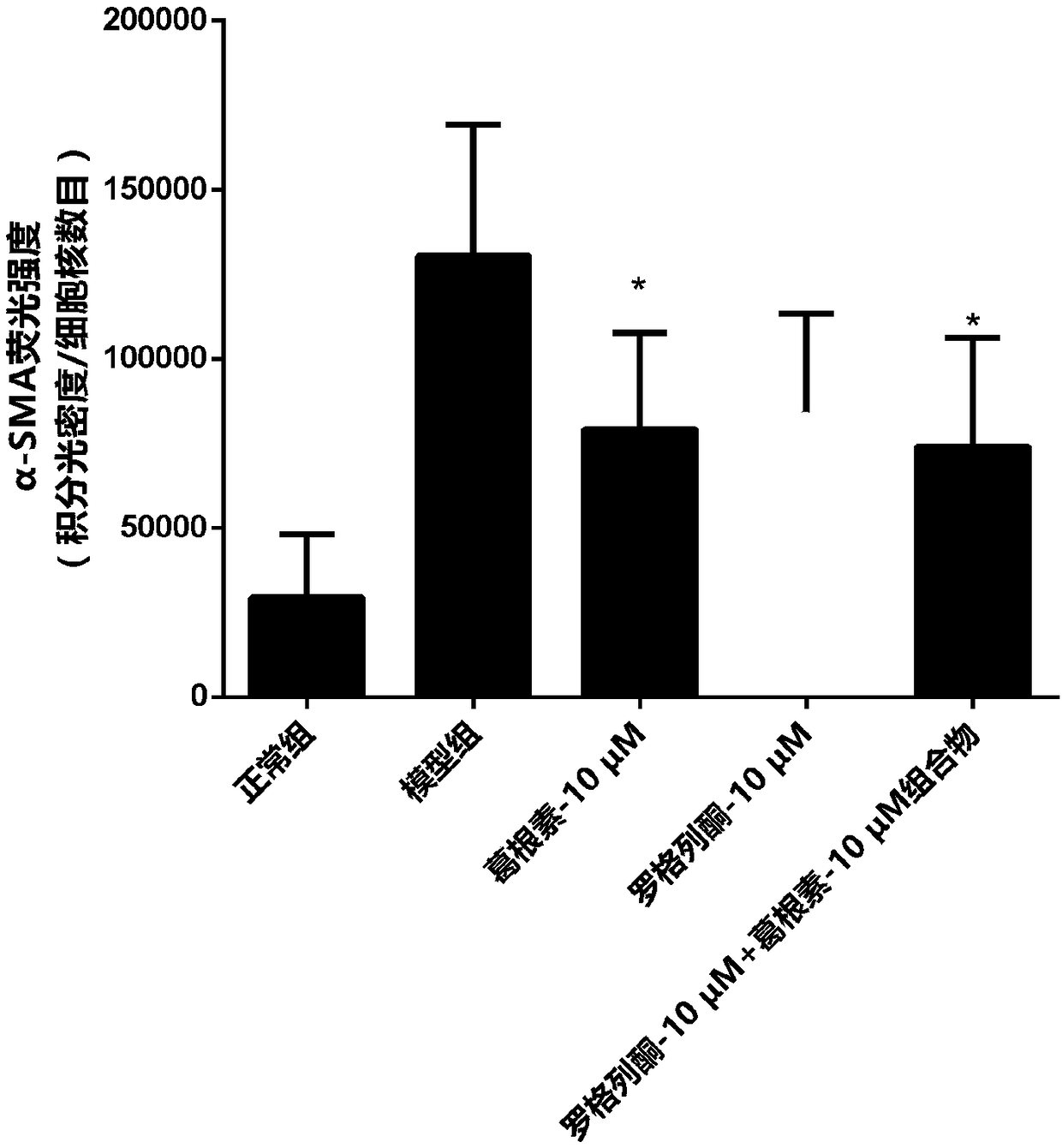
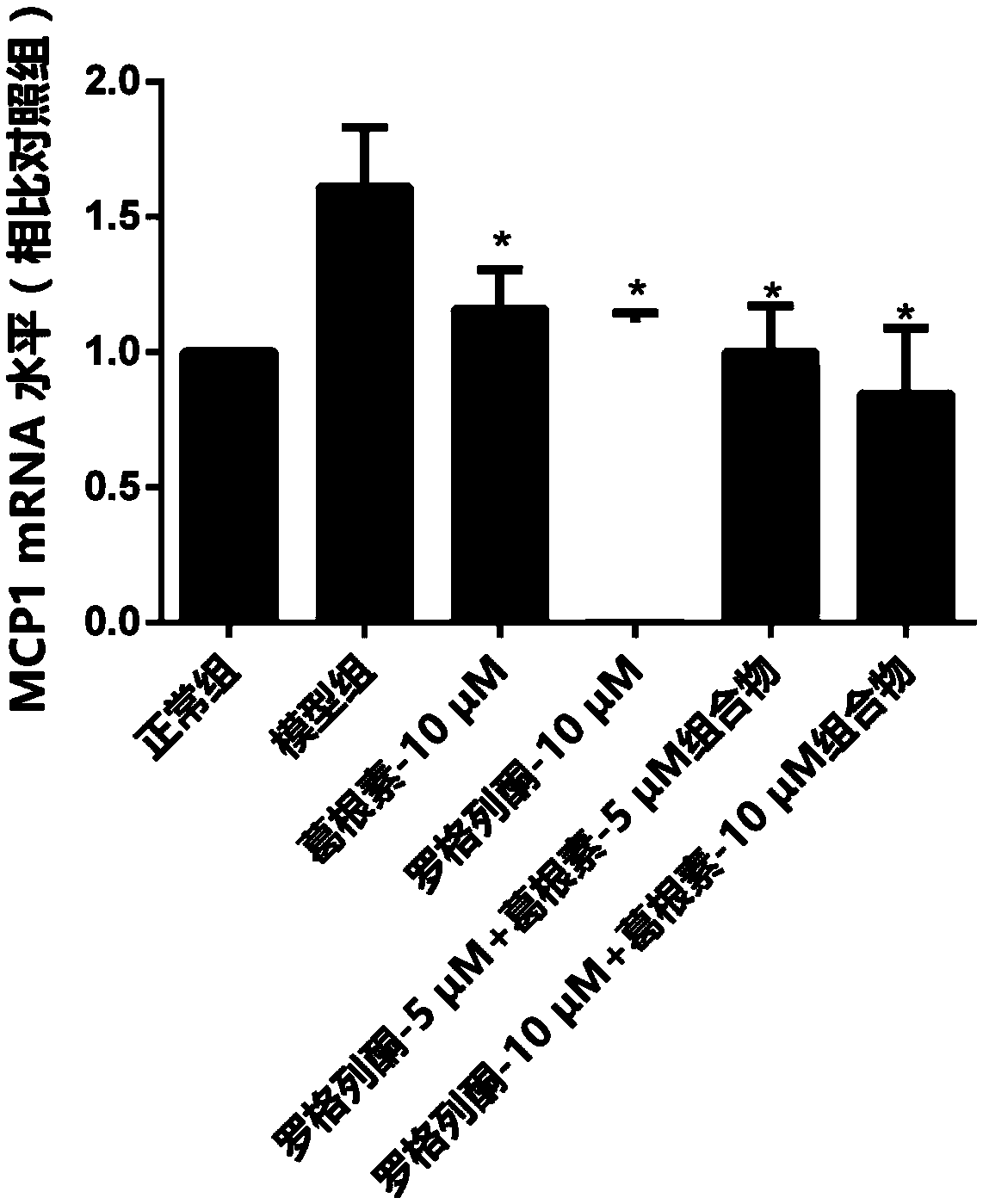
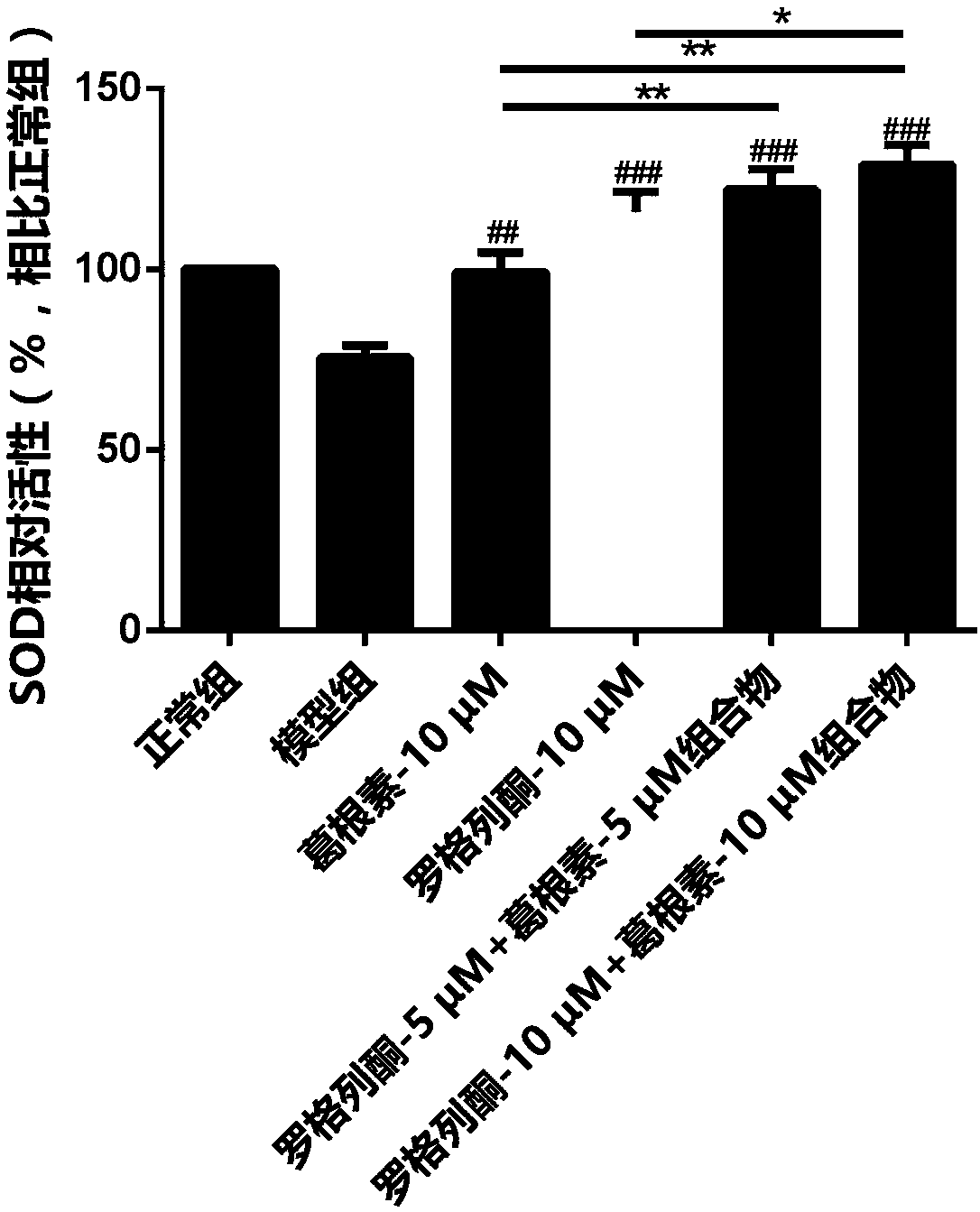
[0031] A control group, a model group, a puerarin treatment group, a rosiglitazone treatment group and a pharmaceutical composition group were set up. In the control group, only 100 μL of low-sugar DMEM culture solution was added, which was regarded as normal culture. I...
Embodiment 2
[0040] 1. Cell culture
[0041] HK-2 cells were cultured in DMEM / F12 medium containing 10% FBS and 1% double antibody, and cultured in a cell incubator. When the cells grew to 70%-80% confluence, they were treated with 0.05% trypsin EDTA solution for digestion. For the experiment, the above-mentioned HK-2 cells were subcultured twice in low-sugar DMEM medium, and then seeded 400,000 cells / well in a 6-well plate. Serum-free double-antibody-free low-glucose DMEM medium was cultured overnight. Afterwards, the old culture medium was discarded and processed in groups.
[0042] 2. Grouping
[0043] A control group, a model group, a puerarin treatment group, a rosiglitazone treatment group and a composition group were set up. Only 100 μL of low-sugar DMEM culture solution was added to the blank group, and 100 μL of 60 mM high-glucose culture solution was added to the other groups, and the drug concentrations contained in the culture solution were shown in Table 2.
[0044] Conta...
Embodiment 3
[0054] 1. Cell culture method
[0055] HK-2 cells were cultured in DMEM / F12 medium containing 10% FBS and 1% double antibody, and cultured in a cell incubator. When the cells grew to 70%-80% confluence, they were treated with 0.05% trypsin EDTA solution for digestion. For the experiment, the above-mentioned HK-2 cells were subcultured twice in low-sugar DMEM medium, and then seeded 400,000 cells / well in a 6-well plate. Serum-free double-antibody-free low-glucose DMEM medium was cultured overnight. Afterwards, the old culture medium was discarded and processed in groups.
[0056] 2. Grouping method
[0057] A control group, a model group, a puerarin treatment group, a rosiglitazone treatment group and a composition group were set up. Only 100 μL of low-sugar DMEM culture solution was added to the blank group, and 100 μL of 60 mM high-sugar culture solution was added to the other groups. The drug concentrations contained in the culture solution are shown in Table 3.
[0058...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com