Method for preparing disubstituted 4-aminocarbazole compound
A technology for aminocarbazoles and compounds, which is applied in the field of preparing disubstituted 4-aminocarbazoles, can solve the problems of severe reaction conditions, inability to obtain 4-aminocarbazoles, and difficulty in obtaining, and achieves the effect of an efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
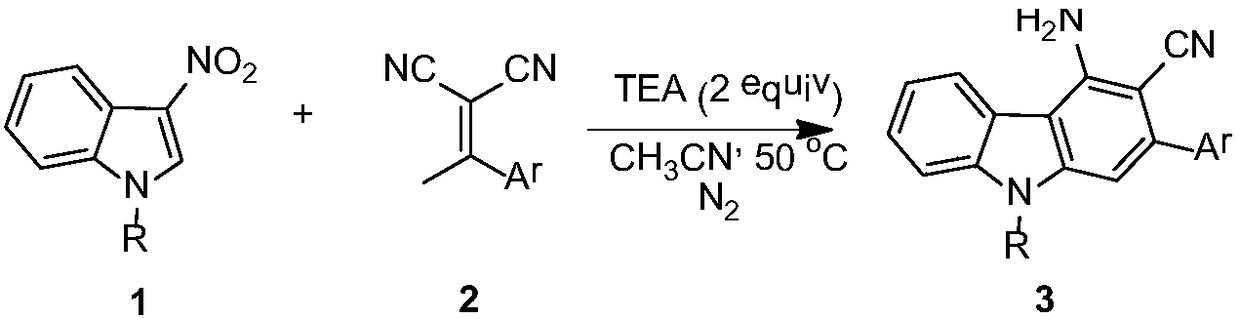
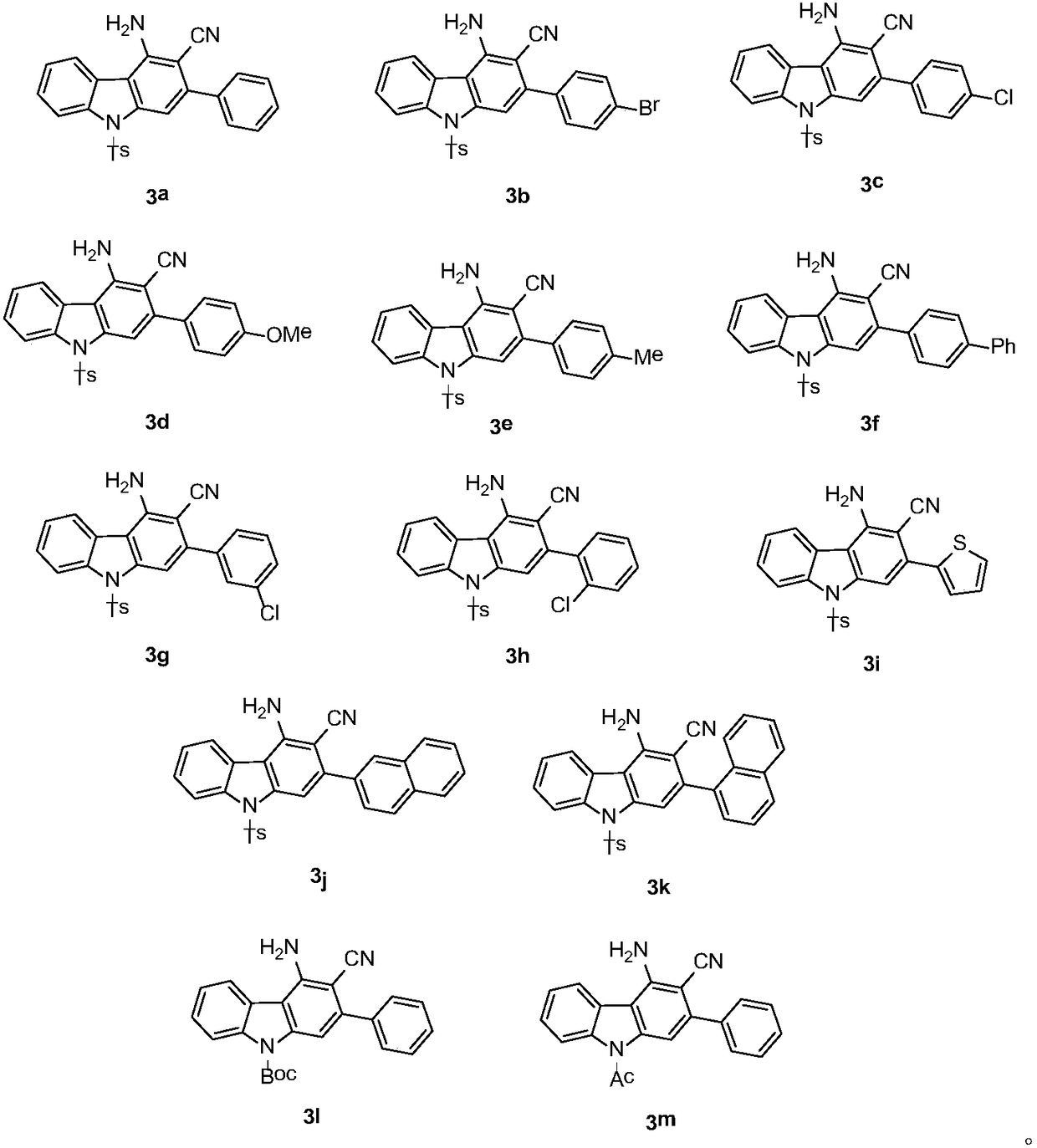
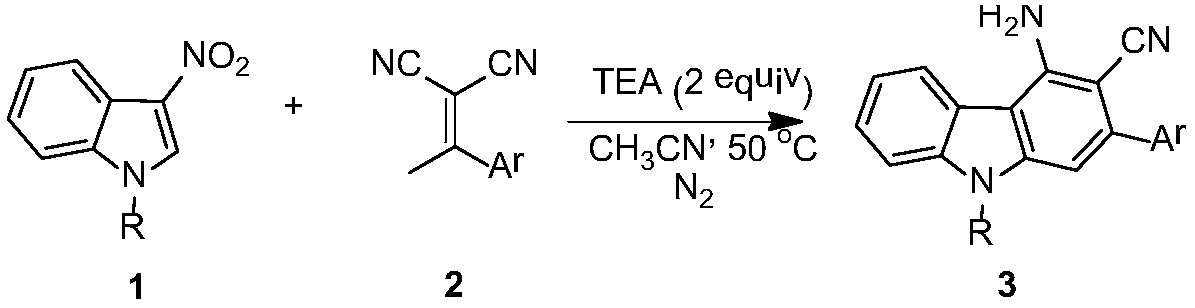
[0014] In a 20 mL dry reaction tube, under nitrogen protection, dissolve 1-p-toluenesulfonyl-3-nitroindole (0.1 mmol) and 2-(1-phenylethylidene) malononitrile (0.2 mmol) Add catalyst triethylamine (0.2mmol) to 2mL of acetonitrile, stir magnetically at 50°C for 24 hours, TLC detects that the raw material disappears, concentrates the organic solvent under reduced pressure, and then further purifies by column chromatography to obtain the product with a yield of 83%;
[0015] 2-Phenyl-3-cyano-4-amino-9-p-toluenesulfonylcarbazole
[0016] 1 H NMR (400MHz, CDCl 3 )δ8.40(d, J=8.4Hz, 1H), 7.88(s, 1H), 7.82(d, J=7.6Hz, 1H), 7.72(d, J=8.4Hz, 2H), 7.64(d, J=7.2Hz, 2H), 7.54-7.42(m, 5H), 7.16(d, J=8.0Hz, 2H); 5.16(brs, 2H), 2.30(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ146.6, 145.5, 144.7, 141.6, 139.0, 138.0, 134.7, 129.9, 128.9, 128.7, 128.6, 126.8, 126.5, 124.5, 124.4, 120.4, 117.6, 115.0, 110.6, 106.3, 91.7: HR, 106.3, 91.7 calcd for[M+H] + (C 26 h 20 N 3 o 2 S) requires 438.1276,...
Embodiment 2
[0018] In a 20mL dry reaction tube, under nitrogen protection, 1-p-toluenesulfonyl-3-nitroindole (0.1mmol) and 2-(1-p-bromophenylethylene)malononitrile (0.2mmol ) was dissolved in 2 mL of acetonitrile, triethylamine (0.2 mmol) was added as a catalyst, magnetically stirred at 50° C. for 6 hours, detected by TLC, the raw material disappeared, the organic solvent was concentrated under reduced pressure, and then further purified by column chromatography to obtain the product with a yield of 68%;
[0019] 2-p-bromophenyl-3-cyano-4-amino-9-p-toluenesulfonylcarbazole
[0020] 1 H NMR (400MHz, CDCl 3 )δ8.41(d, J=8.4Hz, 1H), 7.84-7.82(m, 2H), 7.72(d, J=8.4Hz, 2H), 7.66(d, J=8.4Hz, 2H), 7.56- 7.50(m,3H),7.46(t,J=7.4Hz,1H),7.17(d,J=8.4Hz,2H),5.17(brs,2H),2.31(s,3H); 13 C NMR (100MHz, CDCl 3 )δ146.7, 145.6, 143.4, 141.5, 138.0, 137.9, 134.6, 131.9, 130.5, 130.0, 127.0, 126.5, 124.6, 123.2, 120.4, 117.4, 115.1, 110.8, 106.1, 91.3, 21.6 [M+H] + (C 26 h 19 BrN 3 o 2 S) requires 51...
Embodiment 3
[0022] In a 20mL dry reaction tube, under nitrogen protection, 1-p-toluenesulfonyl-3-nitroindole (0.1mmol) and 2-(1-p-chlorophenylethylidene)malononitrile (0.2mmol ) was dissolved in 2 mL of acetonitrile, added catalyst triethylamine (0.2 mmol), stirred magnetically at 50° C. for 6 hours, detected by TLC, the raw material disappeared, and the organic solvent was concentrated under reduced pressure, and then further purified by column chromatography to obtain the product with a yield of 73%;
[0023] 2-p-chlorophenyl-3-cyano-4-amino-9-p-toluenesulfonylcarbazole
[0024] 1 H NMR (400MHz, CDCl 3 )δ8.41(d,J=8.4Hz,1H),7.85-7.82(m,2H),7.72(d,J=8.4Hz,2H),7.59-7.44(m,6H),7.17(d,J =8.4Hz, 2H), 5.17(brs, 2H), 2.31(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ146.7, 145.6, 143.4, 141.6, 138.0, 137.4, 134.9(3), 134.9(1), 134.6, 130.2, 130.0, 128.9, 127.0, 126.5, 124.6, 124.3, 120.4, 117.4, 115.1, 110.0 ,21.6; HRMS(ESI):calcd for[M+H] + (C 26 h 19 ClN 3 o 2 S) requires 472.0887, found 472....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com