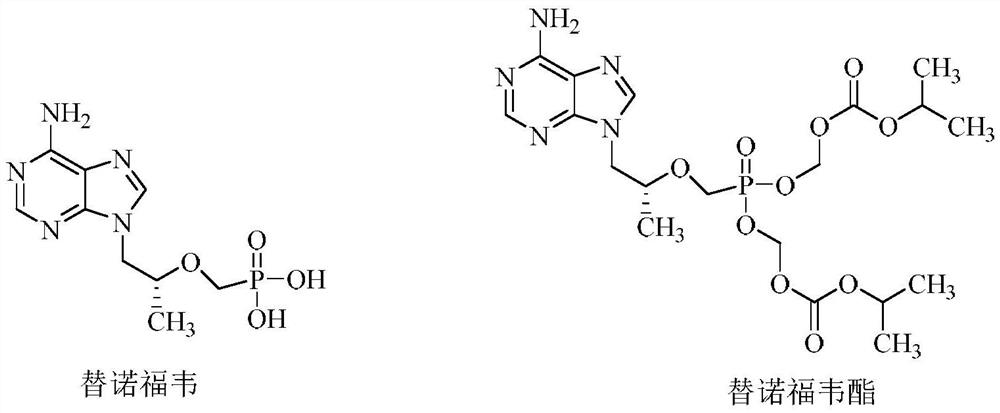
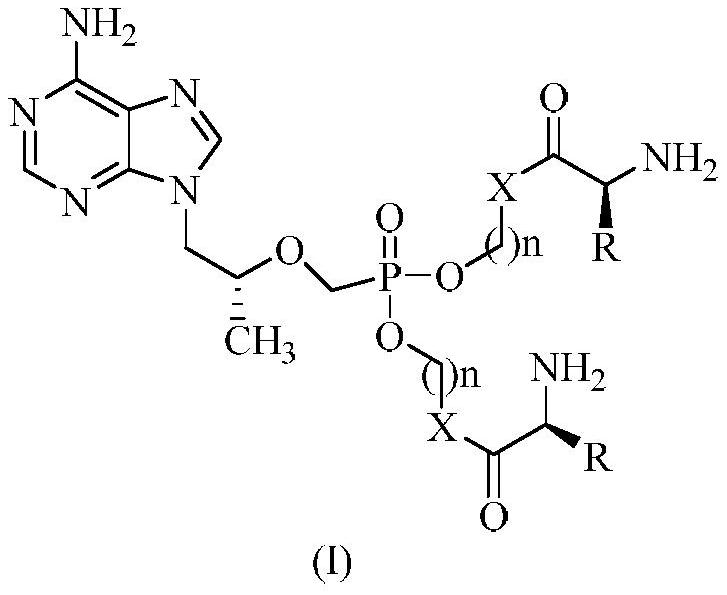
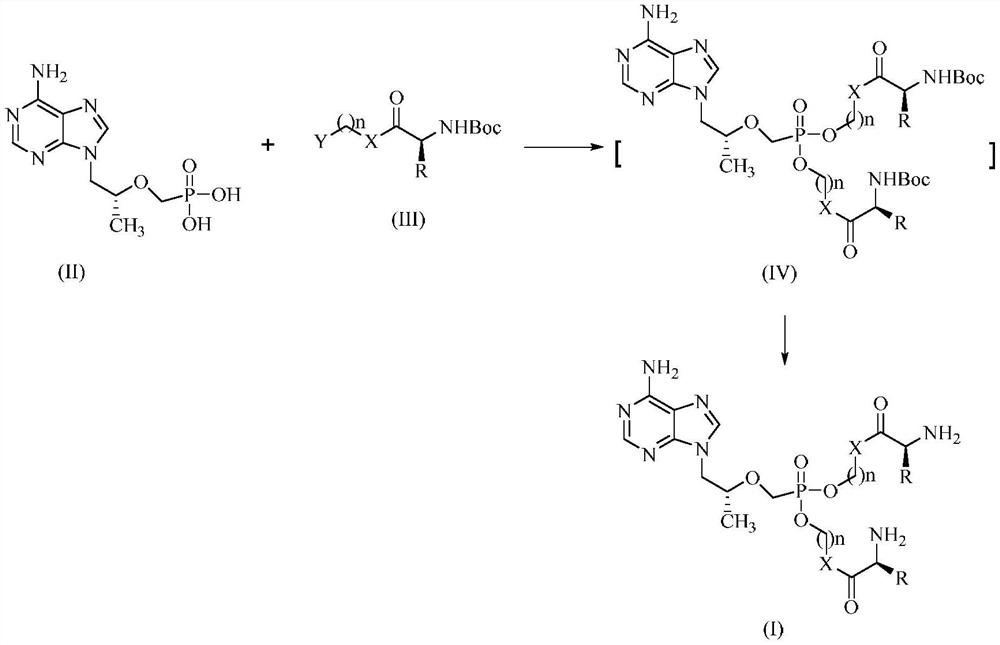
Tenofovir bis-l-amino acid ester and preparation method thereof
A tenofovir and amino acid ester technology, applied in the field of medicinal chemistry, can solve the problems of maintaining sufficient drug concentration at the infection site and poor membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、9
[0062] Embodiment 1, 9-[2-(phosphonomethoxy) propyl] adenine bis-L-valine methyl ester
[0063] Tenofovir (0.50g, 1.74mmol), N-Boc-L-valine-1-bromomethyl ester (1.85g, 6.96mmol) and N,N-dicyclohexyl-4-morpholine amidine ( 1.23g, 4.18mmol) was dissolved in 5mL of dry DMF, stirred at room temperature for 8h, then continued to stir at 80°C for 6h, TLC detected that the reaction of raw materials was complete. Concentrate under reduced pressure, add 30 mL of 1% citric acid to the residue, stir at room temperature for 1 h, extract 3 times with ethyl acetate, combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure to obtain a white foamy solid and It was dissolved in 5mL of dry dioxane, hydrogen chloride gas was passed through, and stirred at room temperature for 4h. Evaporate to dryness under reduced pressure, add ethanol to the residue, stir at 0°C for 15 minutes, then add diethyl ether, continue stirring at 0°C ...
Embodiment 2、9
[0066] Embodiment 2, 9-[2-(phosphonomethoxy) propyl group] adenine bis-L-valine ethyl ester
[0067] The preparation method is the same as in Example 1, tenofovir is reacted with N-Boc-L-valine-2-bromoethyl ester to obtain a white foamy solid.
[0068] 1 H NMR (500MHz, CD 3 OD)8.41(s,1H,8-H),8.37(s,1H,2-H),4.55(d,J=6.0Hz,2H),4.30-4.50(m,10H),3.81-4.09(m ,3H),2.31(m,2H),1.21(m,3H),1.13(d,J=7.0Hz,6H),1.09(d,J=6.5Hz,6H).
[0069] MS-ESI(m / z):574.53(M+H).
[0070] The title compound (70 mg, 0.13 mmol) was dissolved in 5 mL of dry dioxane, hydrogen chloride gas was passed through, and stirred at room temperature for 4 h. Evaporate to dryness under reduced pressure, add ethanol to the residue, stir at 0°C for 15 minutes, then add diethyl ether, continue stirring at 0°C for 30 minutes, filter with suction, rinse the filter cake with a small amount of ether, and dry it in vacuum at 35°C to obtain 9-[2- (Phosphonomethoxy)propyl]adenine bis-L-valine ethyl ester hydrochloride 78 mg,...
Embodiment 3、9
[0076] Example 3, 9-[2-(phosphonomethoxy) propyl] adenine bis-L-leucine propyl ester
[0077] The preparation method is the same as in Example 1, tenofovir is reacted with N-Boc-L-leucine-3-bromopropyl ester to obtain a white foamy solid.
[0078] 1 H NMR (500MHz, CD 3 OD)8.41(s,1H,8-H),8.35(s,1H,2-H),4.52(d,J=7.0Hz,2H),4.30-4.50(m,6H),3.81-4.19(m ,7H),2.35(m,4H),2.20(m,2H),1.25(m,3H),1.22(m,4H),1.08(d,J=7.5Hz,6H),1.01(d,J= 6.5Hz,6H).
[0079] MS-ESI(m / z):630.54(M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com