Engineering bacterium capable of co-producing Danshensu and alanine, and application thereof
A technology of amino acid and transaminase, applied in the field of bioengineering, can solve the problems of complex operation and high cost, and achieve the effect of easy-to-obtain raw materials, simple production process and strong optical specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
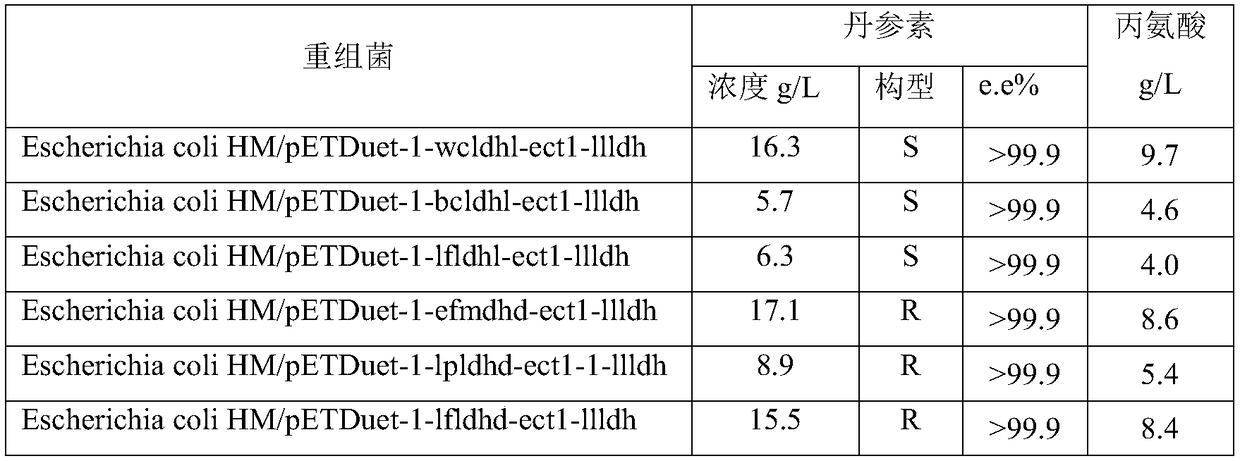
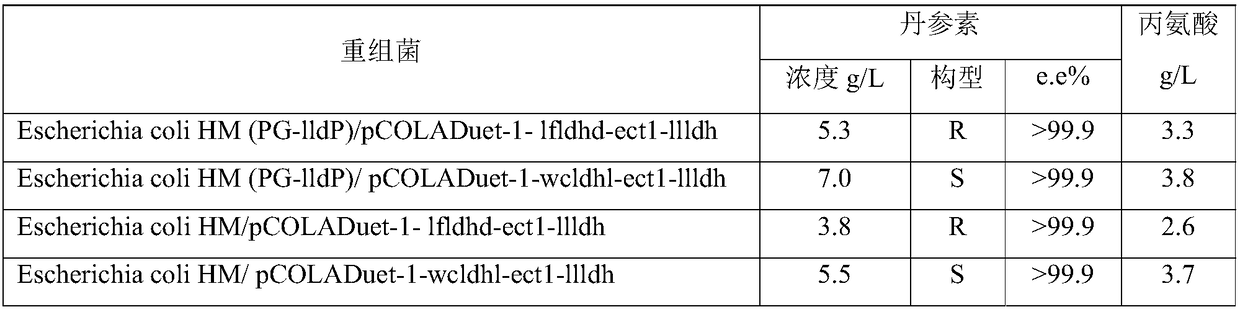
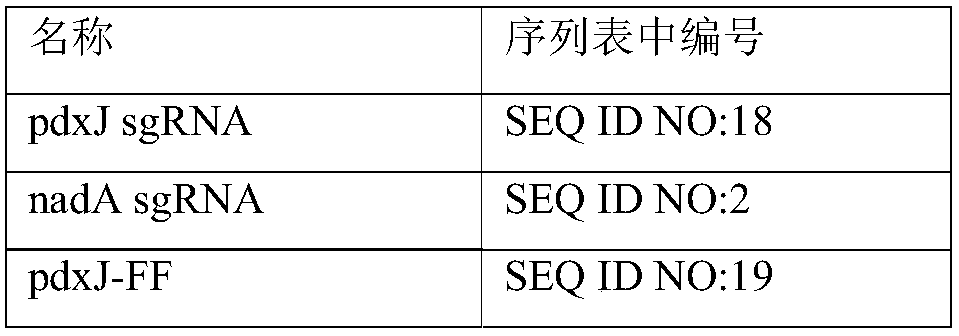
[0067] According to the method described in the literature Large scale validation of an efficient CRISPR / Cas-based multigene editing protocol in Escherichia coli. Microbial Cell Factories, 2017, 16(1):68, hpaD and mhpB on Escherichia coli BL21(DE3) were single-sampled or double knockout. Wherein, the gene knockout plasmid used in the present invention is pCasRed and pCRISPR-gDNA (hpaD sgRNA) and homology arm (hpaDdonor) together into Escherichia coli BL21 (DE3), Cas9 / sgRNA induces the host to produce double-stranded at the hpaD gene site Fragmentation, recombinase Red integrates the hpaD donor into the hpaD gene to achieve gene knockout, and sequence verification. hpaD sgRNA, hpaD donor, mhpB sgRNA, mhpB donor are shown in SEQ ID NO: 16, SEQ ID NO: 17, SEQ ID NO: 25, and SEQ ID NO: 26 in the sequence table, respectively. mhpB was knocked out in the same way.
[0068] Prepare a solution with pH 7, levodopa or D-danshensu 4g / L, wet bacterial volume 200g / L, and measure the conc...
Embodiment 2
[0073] Screening of L-α-amino acid transaminase, a variety of L-α-amino acid transaminase genes were cloned from Escherichia coli and Lactobacillus, and expressed in Escherichia coli BL21 (DE3).
[0074] Induction expression method: The recombinant Escherichia coli was transferred to LB fermentation medium (peptone 10g / L, yeast powder 5g / L, NaCl 10g / L) in a volume ratio of 2%. 600 After reaching 0.6-0.8, IPTG with a final concentration of 0.4 mM was added to induce expression and culture at 20°C for 8 h.
[0075] After induction of expression, cells were collected by centrifugation at 20°C, 8000 rpm, and 20 minutes. The activity of the crude enzyme solution was measured by disrupting the cell, and the activities of various enzymes were compared when pyruvate was used as the ammonia acceptor. 1358.) described the method to measure the activity of L-α-amino acid transaminase, and the results are shown in Table 1. Therefore, it is optimal to select L-α-amino acid aminotransfera...
Embodiment 3
[0079] Comparison of the enzymatic properties of α-hydroxycarboxylic acid dehydrogenases. Usually this type of enzymes may also have the ability to reduce pyruvate to produce lactate, so an enzyme that cannot reduce or very weakly reduces pyruvate is better. Using pyruvate as a substrate, the reducing ability of different enzymes was compared. According to the literature (Serratia marcescens H3010 fermentative D-lactate dehydrogenase gene cloning, expression, purification and enzymatic properties study. Industrial Microbiology, 2012, The method described in 42(04):30-37.) measures the activity of reducing pyruvate with NAD as a coenzyme, and the experimental results are shown in Table 3.
[0080] Table 3 Comparison of pyruvate reduction activities of various α-hydroxycarboxylic acid dehydrogenases
[0081] Recombinant bacteria
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com