Cytotoxicity detection reagent composition
A cytotoxicity and detection reagent technology, applied in the biological field, can solve the problems of poor repeatability, affecting the accuracy and reliability of cytotoxicity detection results, and achieve the effect of reducing interference and improving repeatability and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1, Preparation of CD19CAR-T cells
[0063] The CD19CAR-T cells used in the present invention are prepared according to the method for preparing CAR1920-2-T disclosed in CN105330750A, which is incorporated herein by reference in its entirety.
Embodiment 2
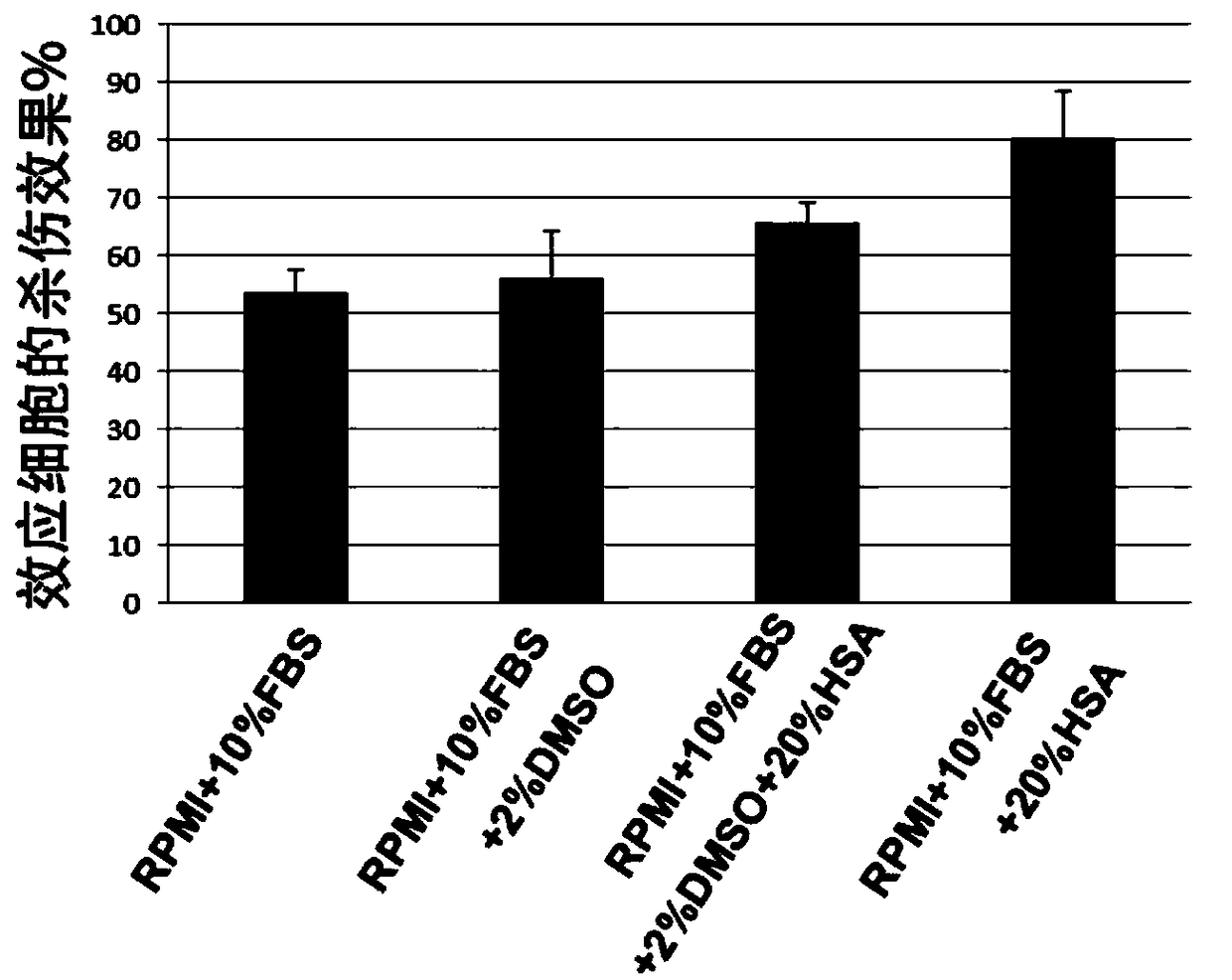
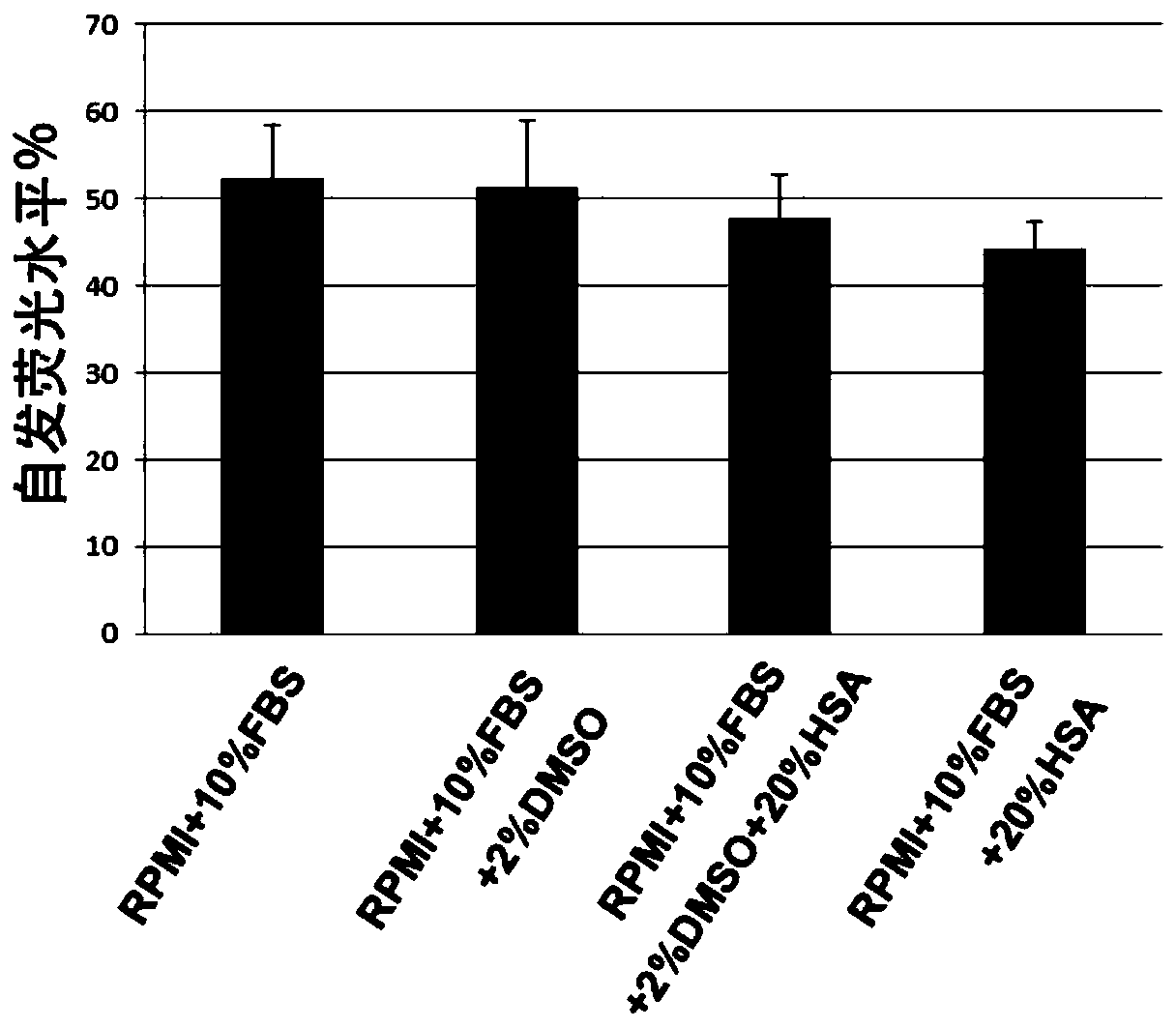
[0064] Example 2. The influence of human serum albumin on the detection effect of CD19CAR-T cells killing target cells
[0065] 1. Preparation of culture medium and detection reagents: The DELFIA lysis buffer in the EuTDA cytotoxicity detection kit was incubated in a 37°C water bath, and the BATDA reagent and DELFIA europium solution were placed at room temperature; the RPMI medium containing 10% FBS was mixed with AIM- V CTS medium was incubated in a 37°C water bath for later use;
[0066] 2. Load the target cells with the fluorescence-enhancing ligand BATDA: place the Raji cell culture medium in a centrifuge at 1500 rpm for 4 minutes, remove the supernatant, and add 1 ml of RPMI serum-free cell culture medium (RPMI+10% FBS) containing 10% FBS to resuspend After counting the cells, adjust the Raji cell density to 1×10 with RPMI medium containing 10% FBS according to the counting results. 6 cells / ml; take 2ml of Raji cells, add 5μl of fluorescence-enhancing ligand (BATDA), ...
Embodiment 3
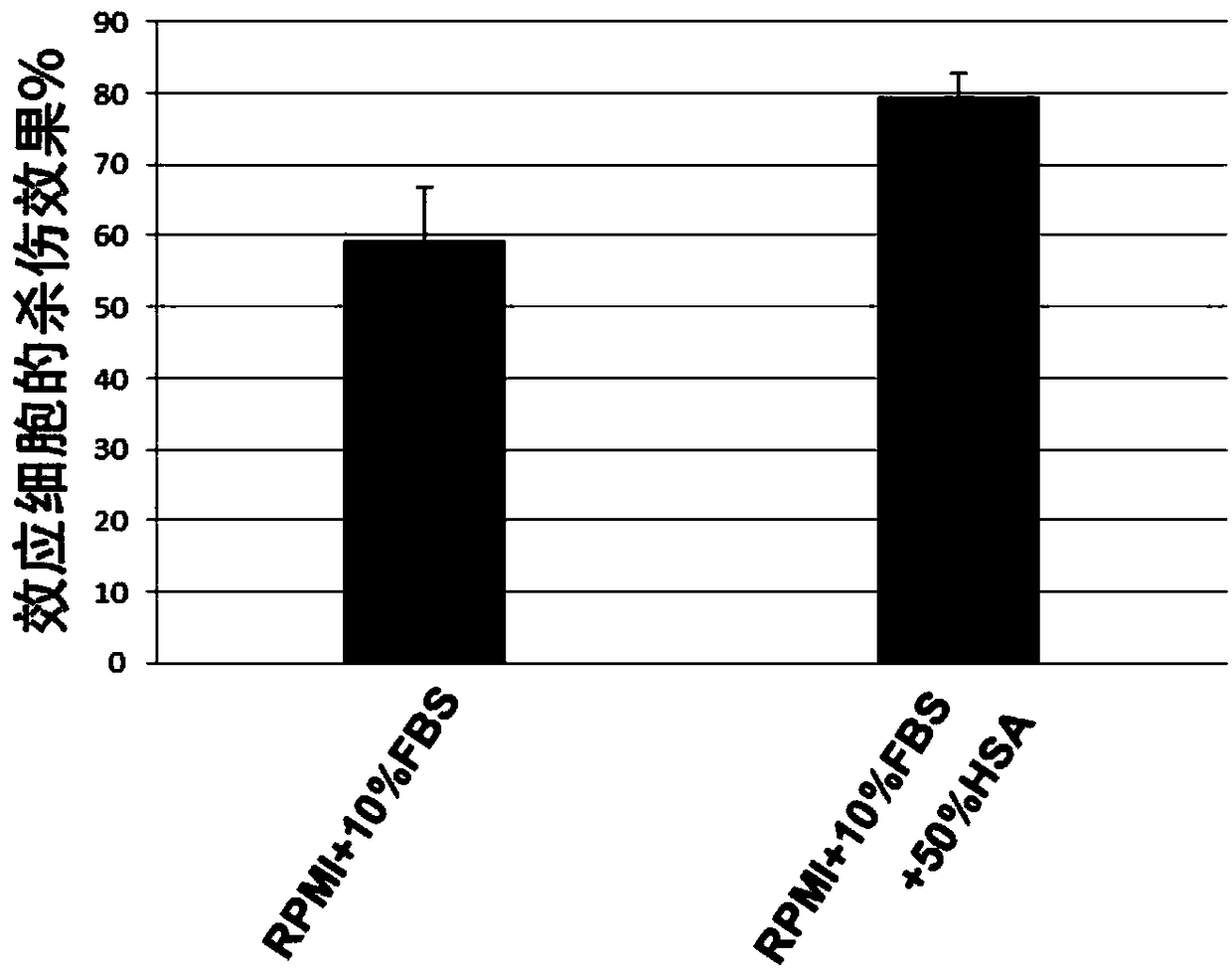
[0079] Example 3. The influence of human serum albumin on the detection effect of CD19CAR-T cells killing target cells
[0080] With reference to Example 2, remove the two groups of samples that added DMSO in the system, only set RPMI+10%FBS and RPMI+10%FBS+50%HSA in step 2, and adjust the effect-target ratio to E:T= 8:1, the rest are the same as in Example 2, and the killing effect and autofluorescence level of CD19CAR-T cells are calculated.
[0081] The result is as image 3 and Figure 4 shown. image 3 It shows the fluorescence level value of the effector cells killing the target cells Raji cells corresponding to the detection results of the killing effect of the CD19CAR-T cells prepared in Example 1 under the two medium conditions of RPMI+10%FBS and RPMI+10%FBS+50%HSA . From image 3 It can be seen that after adding 50% HSA in the culture medium (that is, the final concentration is 25%), the killing effect of the effector cells is significantly increased. And under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com