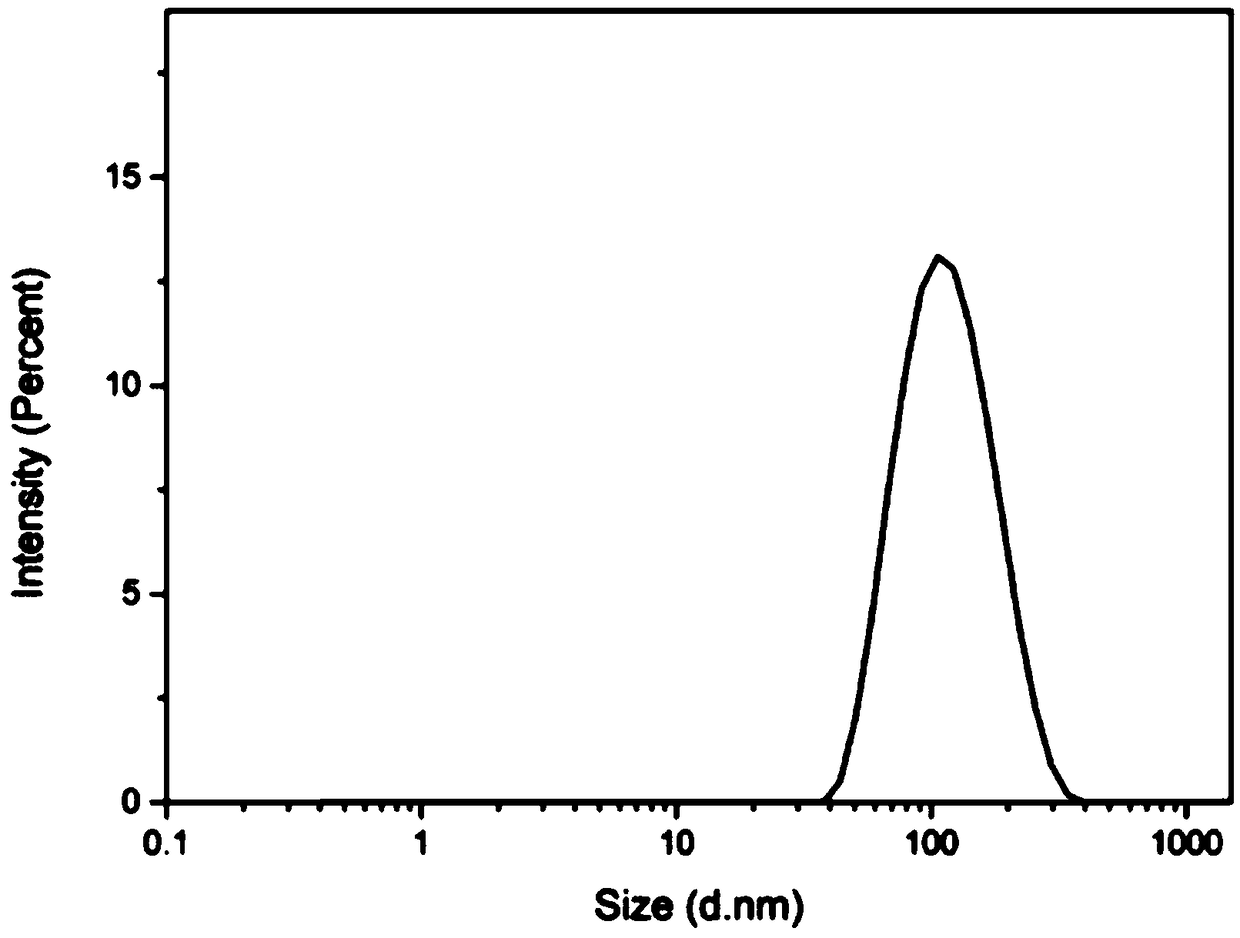
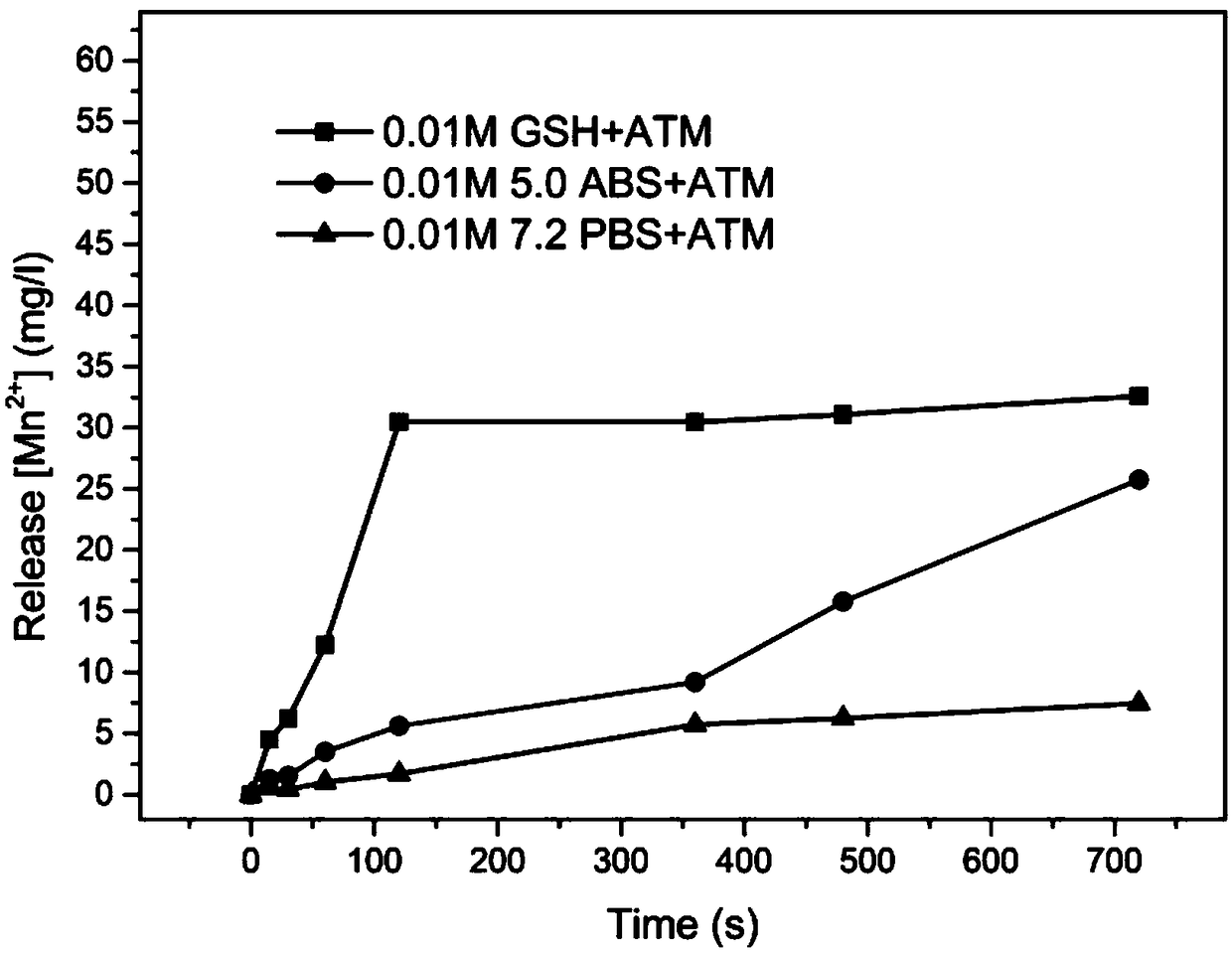
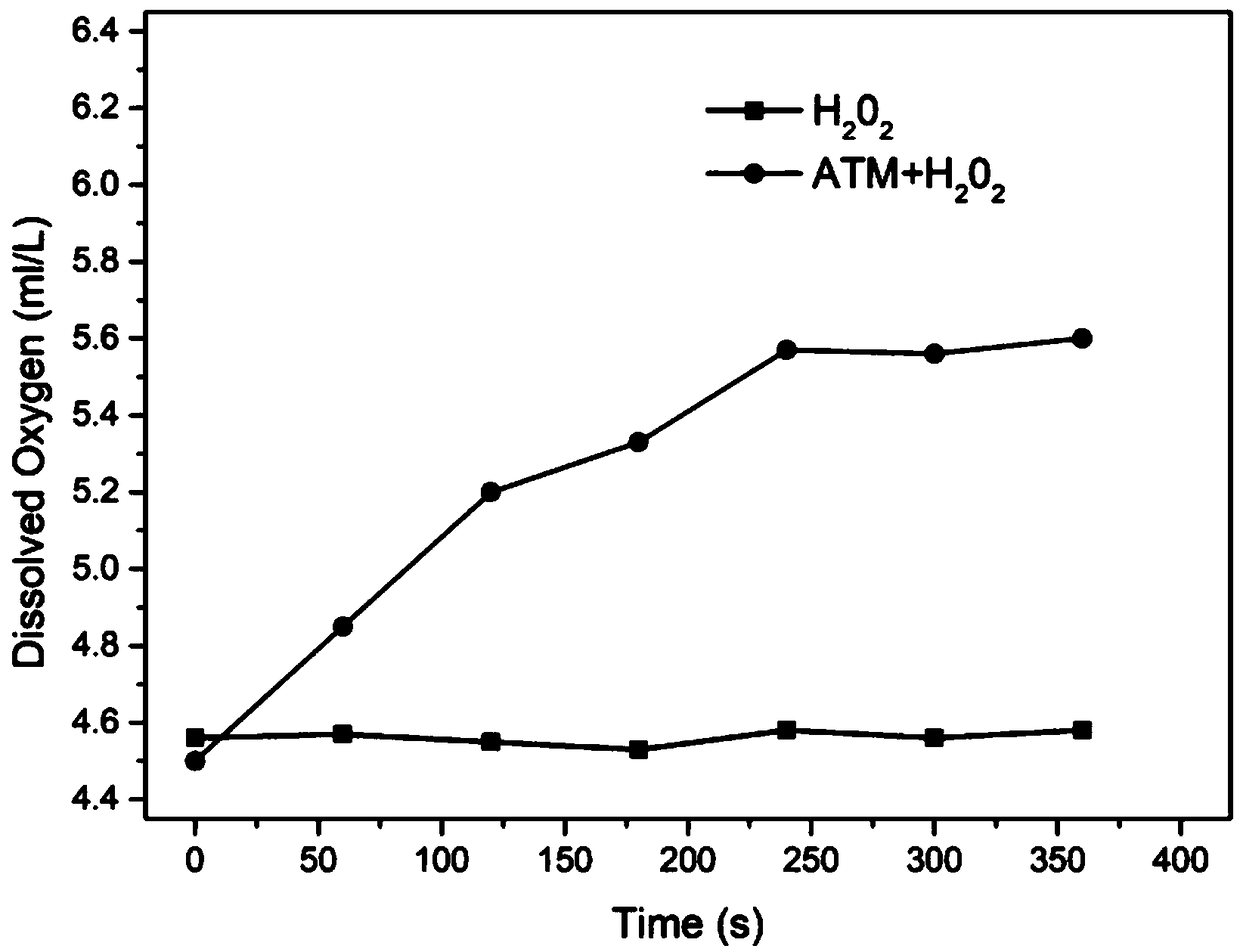
Preparation method of MnO2 carrier coated drug precursor 5-ALA
A technology of 5-ALA and precursors, applied in the field of biomedical materials, to achieve good clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] First dissolve 100mg of 5-ALA in 2ml of deionized water, adjust the pH to 7 and store; then take 200μL of 50mg / ml of 5-ALA, 14mg EDC, 9.3mg NHS, 4.8ml of deionized water and 25ml round bottom flask , React at room temperature for 30min; then add 0.486g BSA, react at room temperature for 12h; add 14mg of MnCl 2 And 4H 2 O was dissolved in 1ml of water and added dropwise to the reaction solution of the above step, and the pH of the reaction solution was adjusted to 11 with 1M NaOH; then transferred to a dialysis bag after 2h, and dialyzed with deionized water for 3 days to obtain the final product. Store in the refrigerator. Mn 2+ MnO is formed under the mineralization of blood bovine albumin 2 Thereby catalyzing endogenous H 2 O 2 Oxygen is generated, and the prepared 5-ALA is used as an intermediate product in the final step of biosynthesis of porphyrin, and is selected as a drug-carrying molecule to perform light action. The blood bovine albumin is used as a stabilizer ...
Embodiment 2
[0024] First dissolve 100mg of 5-ALA in 2ml deionized water, adjust the pH to 7 and store; then take 250μL of 50mg / ml 5-ALA, 14mg EDC, 9.3mg NHS, 4.8ml deionized water and 25ml round bottom flask , React at room temperature for 30min; then add 0.486g BSA, react at room temperature for 12h; add 14mg of MnCl 2 And 4H 2 O was dissolved in 1ml of water and added dropwise to the reaction solution of the above step, and the pH of the reaction solution was adjusted to 11 with 1M NaOH; then transferred to a dialysis bag after 2h, and dialyzed with deionized water for 3 days to obtain the final product. Store in the refrigerator. Mn 2+ MnO is formed under the mineralization of blood bovine albumin 2 Thereby catalyzing endogenous H 2 O 2 Oxygen is generated, and the prepared 5-ALA is used as an intermediate product in the final step of biosynthesis of porphyrin, and is selected as a drug-carrying molecule to perform light action. The blood bovine albumin is used as a stabilizer to minera...
Embodiment 3
[0026] First dissolve 100mg of 5-ALA in 2ml of deionized water, adjust the pH to 7 and store; then take 300μL of 50mg / ml of 5-ALA, 14mg EDC, 9.3mg NHS, 4.8ml of deionized water and 25ml round bottom flask , React at room temperature for 30min; then add 0.486g BSA, react at room temperature for 12h; add 14mg of MnCl 2 And 4H 2 O was dissolved in 1ml of water and added dropwise to the reaction solution of the above step, and the pH of the reaction solution was adjusted to 11 with 1M NaOH; then transferred to a dialysis bag after 2h, and dialyzed with deionized water for 3 days to obtain the final product. Store in the refrigerator. Mn 2+ MnO is formed under the mineralization of blood bovine albumin 2 Thereby catalyzing endogenous H 2 O 2 Oxygen is generated, and the prepared 5-ALA is used as an intermediate product in the final step of biosynthesis of porphyrin, and is selected as a drug-carrying molecule to perform light action. The blood bovine albumin is used as a stabilizer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com