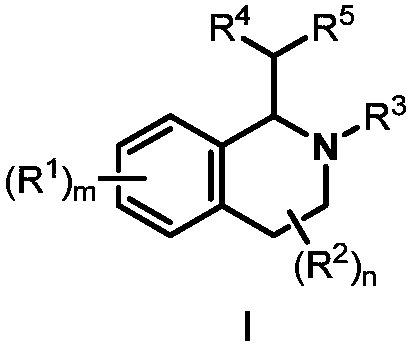
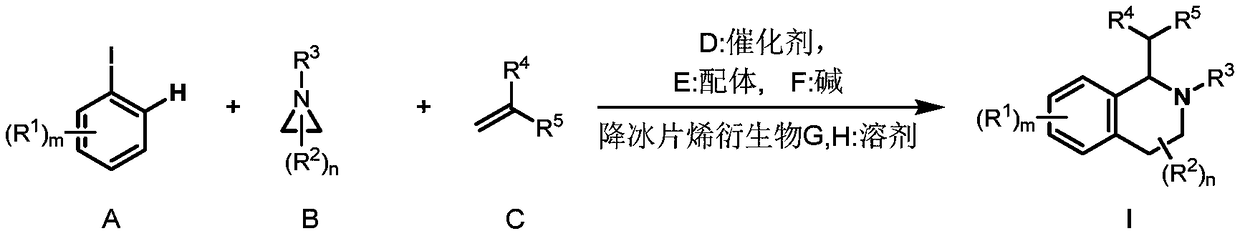
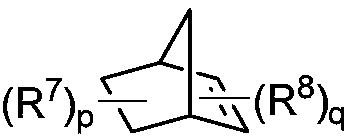
Preparation method of 1,2,3,4-tetrahydroisoquinoline derivative
A technology of tetrahydroisoquinoline and its derivatives, applied in organic chemistry and other fields, can solve problems such as limiting the scope of use of methods, and achieve the effects of low price, good tolerance, universal applicability, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of compound I-1
[0039]
[0040] Under the protection of inert gas, add Pd(OAc) to a dry 4.0mL reaction bottle equipped with a magnetic stir bar 2 (4.6mg, 0.02mmol, 0.10eq), tris(furan-2-yl)phosphine (11.2mg, 0.048mmol, 0.24eq), 2-p-tolyl-3a,4,7,7a-tetrahydro-1H- 4,7-methyleneisoindole-1,3(2H)-dione (10.2mg, 0.04mmol, 0.20 equivalent), potassium carbonate (55.6mg, 0.4mmol, 2.0 equivalent), o-iodotoluene (52.3mg, 0.24mmol, 1.2eq), 1-p-toluenesulfonylaziridine (39.5mg, 0.2mmol, 1.0eq), ethyl acrylate (30.0mg, 0.3mmol, 1.5eq) and dry acetonitrile (1.0mL). The vial was capped and stirred at room temperature for about 5 minutes, after which the mixture was heated to 70°C and stirred for 16 hours. After the reaction vessel was cooled to room temperature, it was quenched with water (10 mL), extracted with ethyl acetate (3×10 mL), Na 2 SO 4 Dry, filter and concentrate in vacuo. Purified by column chromatography, the eluent was petroleum et...
Embodiment 2
[0041] Embodiment 2: the preparation of compound 1-2
[0042]
[0043] The aryl iodide used was 2-ethyliodobenzene (55.7 mg, 0.24 mmol), and other conditions were the same as in Example 1 to obtain compound I-2 (colorless oily liquid, yield 84%). 1 H NMR (400MHz, CDCl 3 ):δ 7.58–7.56(m,2H),7.11–7.01(m,4H),6.77(dd,J=7.5,1.5Hz,1H),5.66(dd,J=10.1,3.8Hz,1H),4.13 (qd,J=7.2,2.6Hz,2H),3.78–3.63(m,2H),2.81–2.69(m,4H),2.65–2.56(m,2H),2.32(s,3H),1.29–1.24 (m,6H). 13 C NMR (100MHz, CDCl 3 ): δ170.23, 143.22, 139.98, 137.28, 133.52, 133.15, 129.40, 127.42, 127.28, 126.85, 126.81, 61.13, 51.09, 42.01, 39.10, 26.77, 24.43, 21.536, 15. Calculated value: C 22 h 27 NaNSO 4 [M+Na + ] 424.1553, measured value: 424.1556.
Embodiment 3
[0044] Embodiment 3: the preparation of compound 1-3
[0045]
[0046] The aryl iodide used was 2-isopropyl iodobenzene (59.1 mg, 0.24 mmol), and other conditions were the same as in Example 1 to obtain compound I-3 (colorless oily liquid, yield 86%). 1 H NMR (400MHz, CDCl 3 ): δ 7.59–7.56(m,2H),7.13–7.07(m,4H),6.74(dd,J=6.4,2.4Hz,1H),5.73(dd,J=10.3,3.9Hz,1H),4.19 –4.11(m,2H),3.76–3.67(m,2H),3.23–3.17(m,1H),2.80 (dd,J=14.6,10.2Hz,1H),2.73–2.69(m,2H),2.55 (dd,J=14.6,3.9Hz,1H),2.31(s,3H),1.30–1.24(m,6H),1.22(d,J=6.7Hz,3H). 13 C NMR (100MHz, CDCl 3):δ 170.13,144.97,143.19,137.34,133.00,132.57,129.38,127.57,127.27,126.79, 124.01,61.12,50.97,42.49,38.98,27.90,26.87,25.26,23.49,21.54,14.29.HRMS (ESI-TOF ): Theoretical calculation value: C 23 h 29 NaNSO 4 [M+Na + ] 438.1710, measured value: 438.1712.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com