Specific yolk immunoglobulin composition and preparation thereof
A technology of egg yolk immunoglobulin and composition, which is applied in the field of specific egg yolk immunoglobulin composition and its preparation to achieve good killing effect, solve the problem of pan-drug resistance, and have the effects of strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] Further, the preparation method of the specific yolk immunoglobulin composition comprises:
[0017] S1. Mix the inactivated Acinetobacter baumannii, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, cluster A and type B hemolytic streptococcus evenly to make a composite antigen.
[0018] Wherein, according to parts by weight, the composite antigen includes 20 to 25 parts of inactivated Acinetobacter baumannii, 15 to 20 parts of Staphylococcus aureus, 10 to 15 parts of Escherichia coli, 10 to 15 parts of Pseudomonas aeruginosa, 10 to 15 copies of Candida albicans, 25 to 35 copies of cluster A and type B hemolytic streptococci.
[0019] Preferably, in parts by weight, the composite antigen includes 21-23 parts of inactivated Acinetobacter baumannii, 17-20 parts of Staphylococcus aureus, 12-14 parts of Escherichia coli, and 12-14 parts of Pseudomonas aeruginosa , 10 to 13 copies of Candida albicans, 28 to 32 copies of cluster A and type B ...
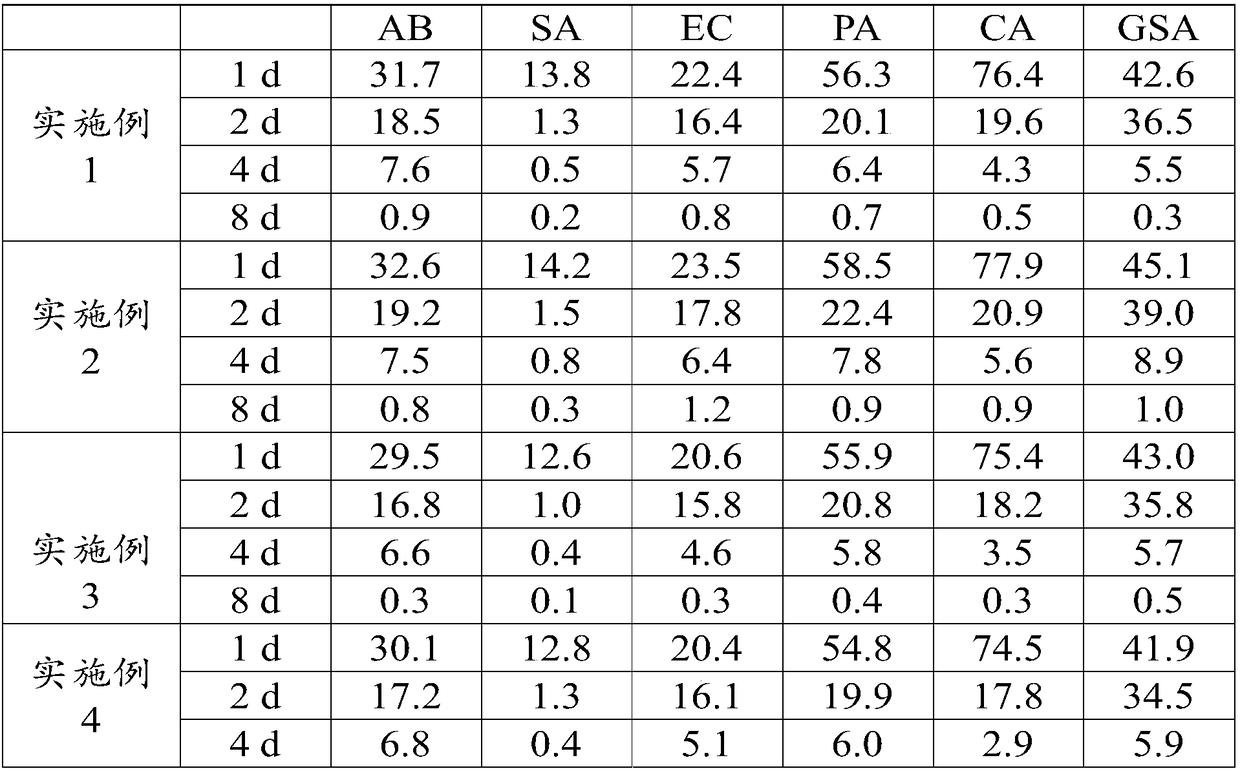
Embodiment 1
[0039] This embodiment provides a specific yolk immunoglobulin composition, the preparation method of which is as follows:
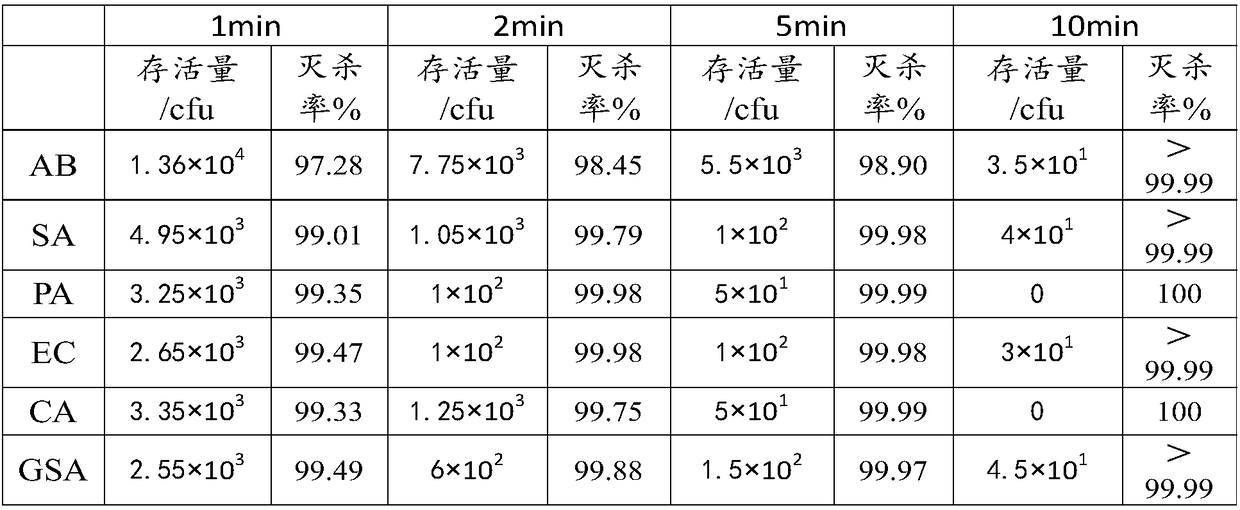
[0040] S1. Select six pathogenic bacteria, Acinetobacter baumannii, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, group A and type B hemolytic streptococcus, and use LB medium at 38°C respectively Cultivate in a three-gas incubator for 18 hours; then separate the above strains and inoculate them into broth medium, and cultivate them in a constant temperature double-layer shaking incubator at 38°C for 23 hours. The speed of the constant temperature shaking incubator is 200rpm. Then inactivate it for later use.
[0041] The six pathogenic bacteria after inactivation treatment were divided into 25 parts of Acinetobacter baumannii, 20 parts of Staphylococcus aureus, 10 parts of E. Mix 25 parts of streptococcus sexually to obtain a strain mixture.
[0042] Dissolve the mixed strain mixture in 0.1mol / L PBS solution to pre...
Embodiment 2
[0046] This embodiment provides a specific yolk immunoglobulin composition, the preparation method of which is as follows:
[0047] S1. Select six pathogenic bacteria, Acinetobacter baumannii, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, group A and type B hemolytic streptococci, and use LB medium at 37°C respectively Cultivate in a three-gas incubator for 20 hours; then separate the above strains and inoculate them into broth medium, and culture them in a constant temperature double-layer shaking incubator at 37°C for 28 hours, and the speed of the constant temperature shaking incubator is 300rpm. Then inactivate it for later use.
[0048] The six pathogenic bacteria after inactivation treatment were divided into 20 parts of Acinetobacter baumannii, 15 parts of Staphylococcus aureus, 15 parts of E. Mix 35 parts of streptococci to obtain a strain mixture.
[0049] Dissolve the mixed strain mixture in 0.2mol / L PBS solution to prepare the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com