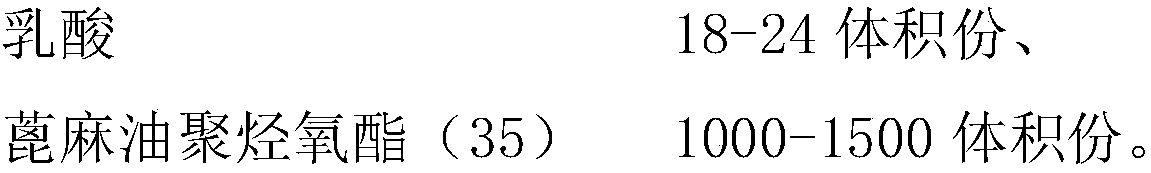Preparation method of paclitaxel injection
A paclitaxel and injection technology, applied in the field of biomedicine, can solve problems such as hypersensitivity, and achieve the effects of low production energy consumption, high production efficiency, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: A paclitaxel injection
[0021]
[0022] The preparation method is:
[0023] A. Prepare the materials according to the production prescription, and weigh the original and auxiliary materials according to the prescription and reserve;
[0024] B. Add one-third of the prescription amount of absolute ethanol to paclitaxel, ultrasound, add four-fifths of the prescription amount of polyoxyethylene castor oil, and one-half of the prescription amount of lactic acid, and mix thoroughly;
[0025] C. Pre-freeze the solution at -50°C for 3 hours; then sublimate and dry at -15°C for 12 hours; then increase the temperature to 30°C for re-drying, for 6 hours, pre-freeze, sublimate and dry, and then Paclitaxel freeze-dried powder is obtained in the drying stage;
[0026] D. Take the paclitaxel lyophilized powder obtained in step C, add the remaining amount of absolute ethanol to ultrasonically dissolve, add the remaining amount of polyoxyethylene castor oil and the remaining amount ...
experiment example 1
[0040] Experimental Example 1 Stability Comparison Study Results
[0041] The paclitaxel injection in Example 1 of the present invention and the products prepared in Comparative Example 1 and Comparative Example 2 were subjected to accelerated tests and long-term tests to determine various indicators.
[0042] Accelerated test
[0043] Take trial samples and place them at 40℃±2℃ / RH75±5% for 6 months for accelerated test investigation. Samples were taken at the end of the first 1, 2, 3, and 6 months, and the inspection items were tested according to the inspection method of the national standard for paclitaxel injection WS1-(X-026)-2001Z, and the results were compared with those in 0 months. The results are as follows in Table 1:
[0044]
[0045] Long-term test Take trial samples and place them under the conditions of temperature 25℃±2℃ and relative humidity 60%±10% for long-term test. Samples were taken at the end of the 0th and 6th months, and the inspection items were tested acco...
experiment example 2
[0050] Experimental example 2 Allergy test
[0051] The injection prepared in Example 1 of the present invention and the injection in Comparative Examples 1-2 were used for animal experiments, and converted into the same effective amount of paclitaxel before use.
[0052] Allergy test method: Take 18 guinea pigs and randomly divide them into three groups. Inject 6mg / mL paclitaxel injection into the intraperitoneal cavity at a dose of 3mg / head, once every other day, three times in a row, on the 14th and 21st days after the first injection. The group took 3 guinea pigs each time and challenged them by intravenous injection of 6 mg / mouse, and judged the allergic reaction score according to Table 4's systemic allergic reaction rating standard.
[0053]
[0054]
[0055] The results are shown in Table 5 (average value):
[0056]
[0057] From the results in Table 5, it can be seen that the allergic reaction of paclitaxel injection of the present invention is between 0-1 grade, and the alle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com