1-(2,5-dimethoxyphenyl)-3-(substituted pyrimidine-4-yl)urea compound and preparation and application thereof
A dimethoxyphenyl compound technology, applied in the field of medicinal chemistry, can solve problems such as adverse reactions, inconvenient medication for patients, and toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 compound
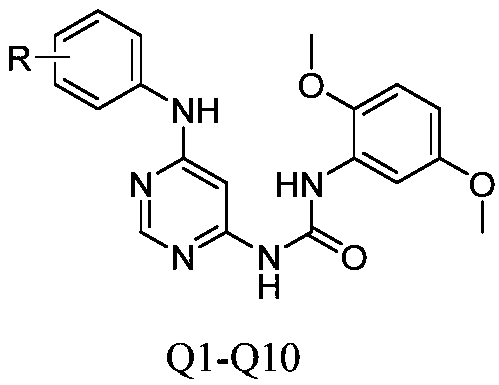
[0029] 1.1 The specific synthetic route of the compound is as follows:
[0030]
[0031] 1.2 Synthesis steps
[0032] a. the synthesis of the first step intermediate product:
[0033] Step 1: Take a dry three-neck reaction bottle and put it into a magnet. Add 4-amino-6-chloropyrimidine (1eq), KI (0.5eq), and dissolve with absolute ethanol (35mL). After heating with stirring for 10 min on a magnetic stirrer, trifluoroacetic acid (200 mL) was added. activation. After about 1 h, substituted aniline (0.8 eq) dissolved in absolute ethanol (15 mL) was added for reaction. Note that it should be added in a dropwise manner to achieve the effect of long-term excess reaction, and the dropping time should be controlled at about 1 hour.
[0034] Step 2: use TLC method to detect the reaction progress and reaction effect. Generally, after 36 hours of reaction, the reaction is almost complete. After the reaction is complete, first spi...
Embodiment 2
[0055] Example 2 compound anti-tumor cell activity
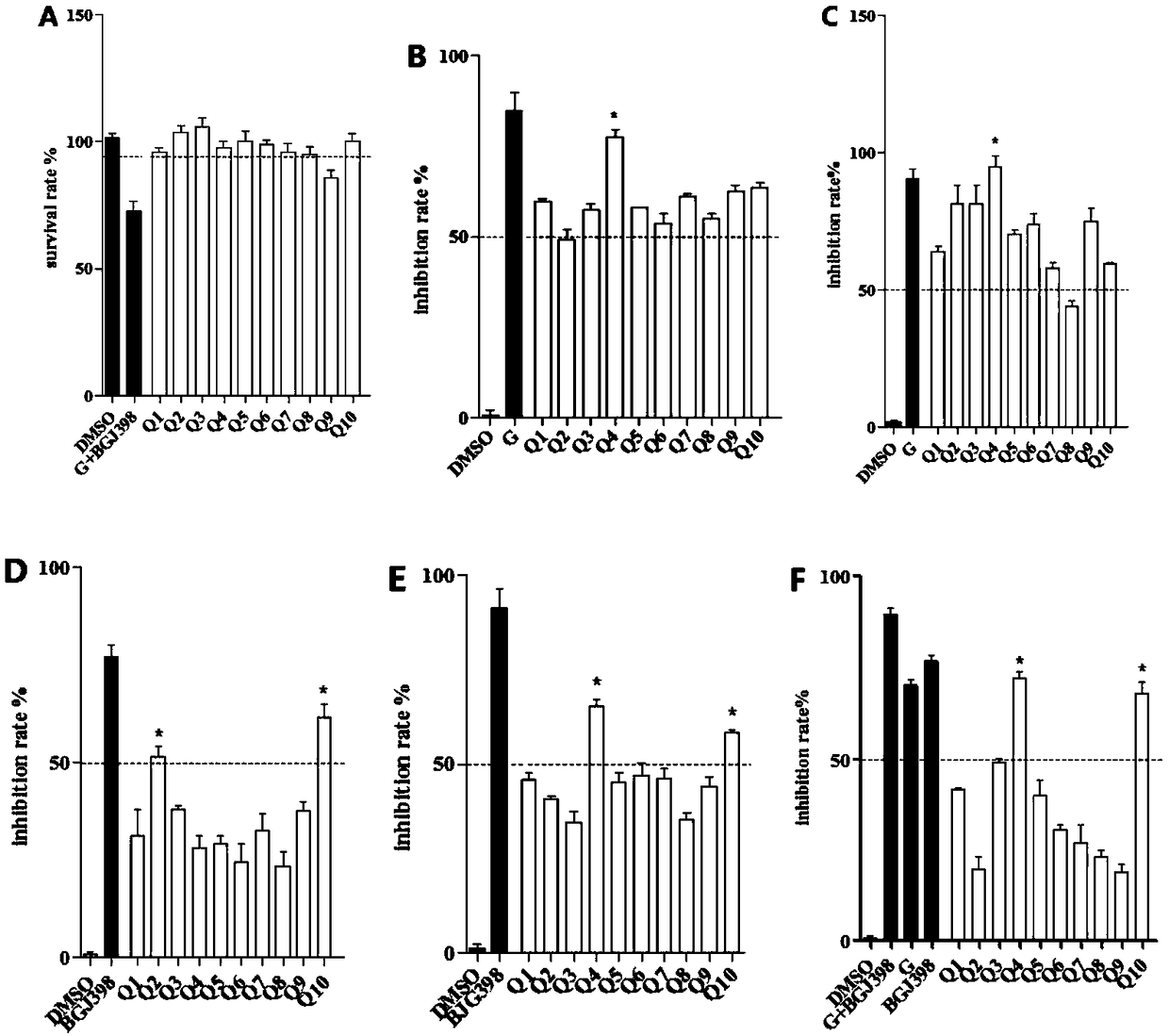
[0056] 2.1 Antitumor activity of compounds tested by MTT method
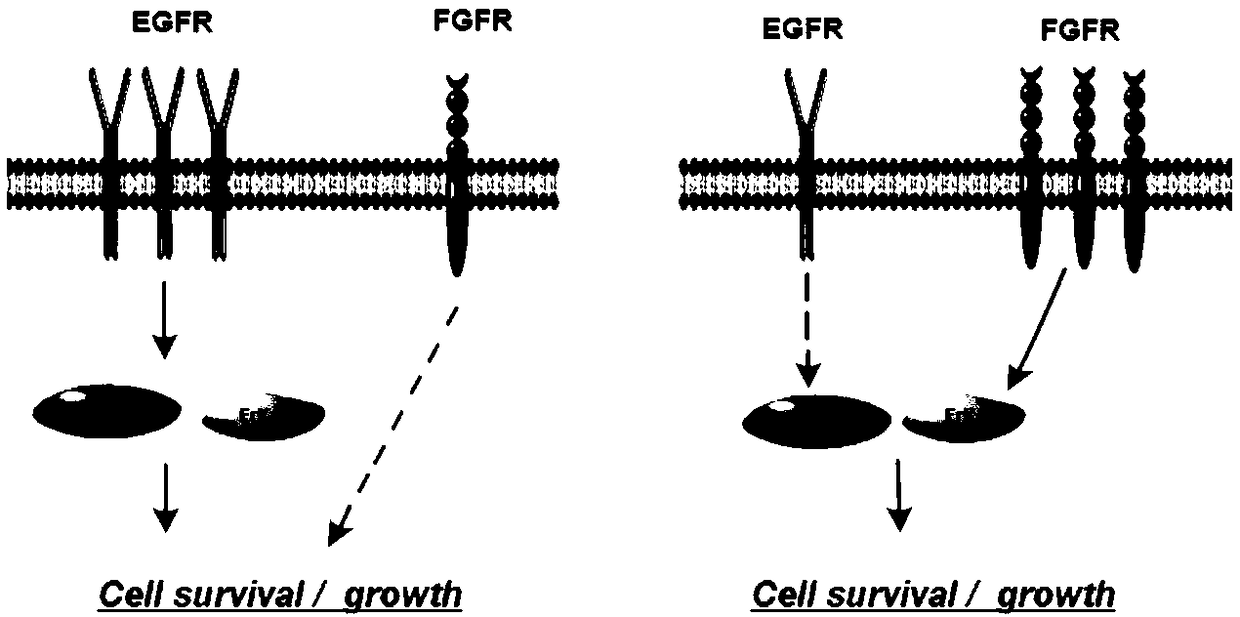
[0057] This experiment uses the MTT method. The selected normal lung cells were BEAS-2B cells; the selected five non-small cell lung cancer cells included lung adenocarcinoma cell A549 (WT EGFR), lung adenocarcinoma cell PC-9 (EGFR d746-750), lung squamous cell carcinoma Cancer cell H520 (FGFR amplification), large cell lung cancer H1581 (FGFR amplification) and lung squamous cell carcinoma H226 (FGFR amplification / overexpressed EGFR). The above-mentioned cells with logarithmic growth were selected, digested, collected, and counted with a cell counting plate. Next, dilute the counted cells to an appropriate concentration (5*10^4 cells / mL~8*10^4 cells / mL), and add the diluted cell suspension to the 96-well plate at 100 μL per well and remember to set blank control wells containing only medium on the same well plate; after plating overnight, replace with fres...
Embodiment 3
[0060] IC of embodiment 3 compound Y6 and Y13 to five kinds of tumor cells 50 experiment
[0061] 3.1 IC of compounds tested by MTT method 50 value
[0062] According to the results of the toxic effects of all the target compounds on normal lung cells and the results of the inhibitory effects on five NSCLC cell lines, we obtained compound Q10 which has inhibitory effects on all five NSCLC cell lines. We choose this compound, further to do IC 50 experiment. Six concentrations (20 μM, 10 μM, 1.0 μM, 0.50 μM, 0.10 μM and 0.01 μM) were first set for this compound. Then, five non-small cell lung cancer cell lines, including A549 cells, PC-9 cells, H520 cells, H1581 cells and H226 cells, were treated with different concentrations of this compound. Obtain the optical density (OD) value, calculate the inhibition rate, and calculate the IC of this compound by GraphPad Prism 5 software 50 value.
[0063] 3.2 Experimental results
[0064] IC for five cancer cells: A549 cells, PC-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com