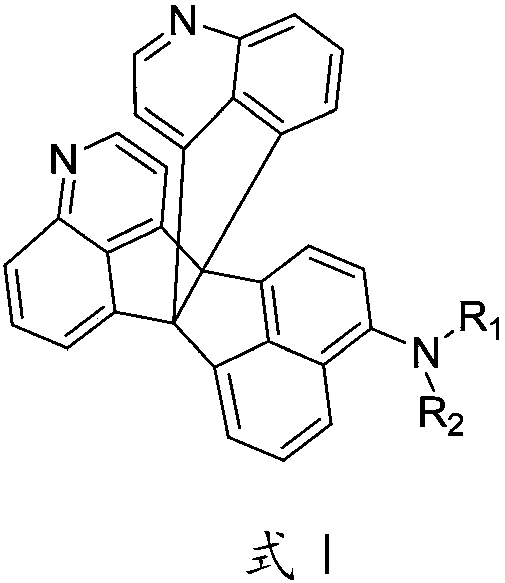
Organic photoelectric material as well as preparation method and application thereof
An organic photoelectric material and organic technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of device efficiency attenuation, expensive phosphorescent materials, unstable blue light materials, etc., to reduce the driving voltage and increase the loading Effects of carrier mobility, luminous efficiency, and improvement of photoelectric characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
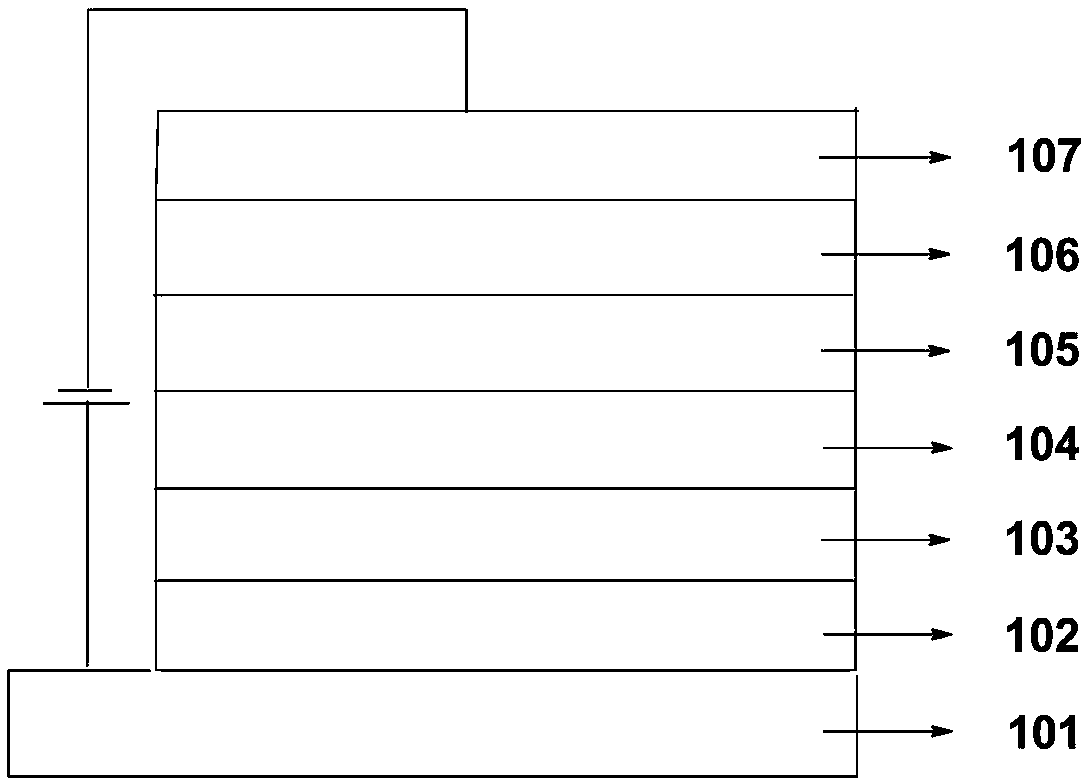
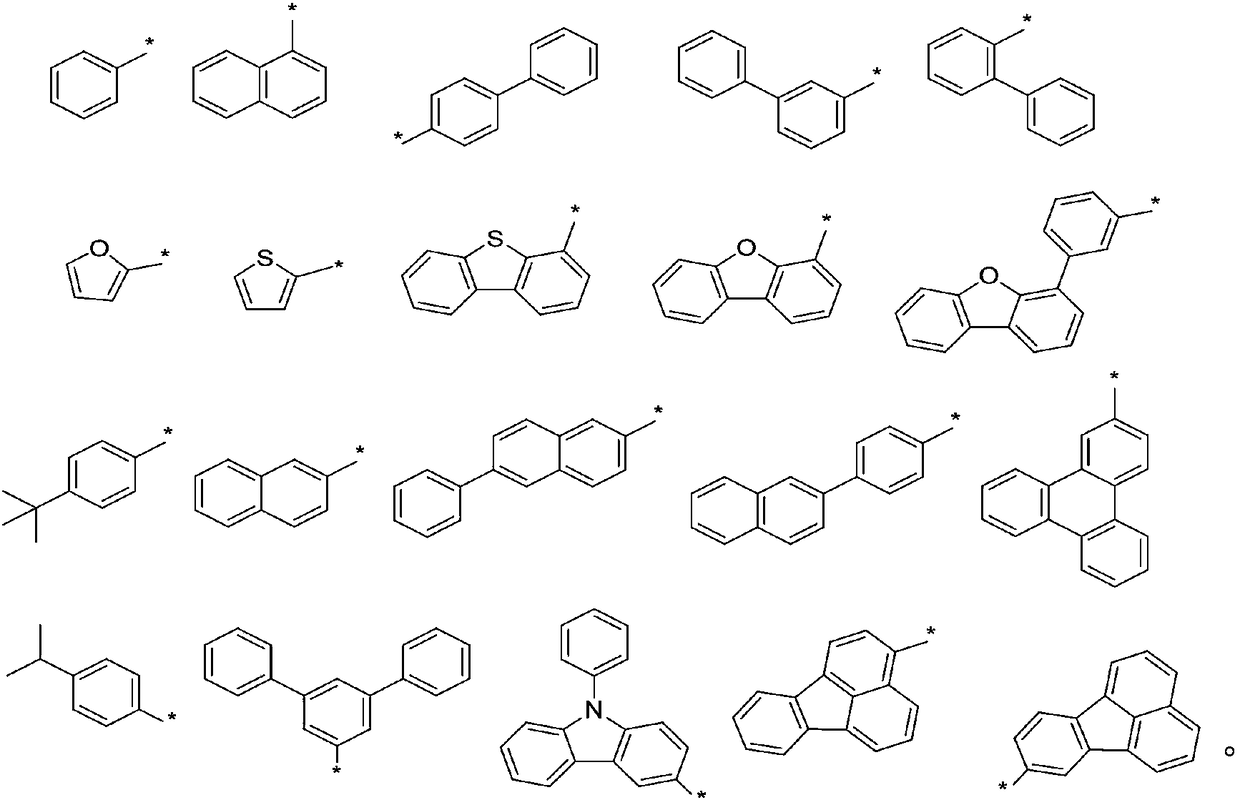
Image
Examples
Embodiment 1
[0044] Embodiment 1: Preparation of aforementioned materials
[0045] Step (1) Preparation of Intermediate A1
[0046]
[0047] Sequentially weigh 100.0g (0.55mol) of acenaphthenequinone and 500g of tetrahydrofuran in a 2L three-necked bottle, and lower the temperature to -20°C under nitrogen protection, and add 281.2g (1.21mol) of 4-quinolylmagnesium bromide dropwise to the above-mentioned bottle. Tetrahydrofuran solution (tetrahydrofuran is 500g), 1.0hrs is added dropwise, and after insulation-20 ℃ of reaction 2.0hrs, by TLC follow-up reaction until no raw material is left, add 400g mass fraction in reaction system and be 10% hydrochloric acid quenching reaction, reduce After removing the solvent by pressure, add 1000 g of ethyl acetate and 500 g of water to the above system, separate the organic phase, wash the organic phase with 500 g of water each time, wash with water for 3 times, and pass through a silica gel column. Use 100g of ethyl acetate and 500g of petroleum e...
Embodiment 2
[0057] Embodiment 2: prepare aforementioned material C02
[0058] The same preparation method and raw material ratio as in the preparation of material C01 in Example 1 were adopted, except that diphenylamine was replaced by bis(4-isopropylphenyl)amine in step (4), and the rest remained unchanged. The material C0 finally obtained is detected through high-performance liquid chromatography (being called for short HPLC) to know that the purity of material C02 is 99.65%, and it is known through high-resolution mass spectrometry that the theoretical value [M + ] is 655.2987 and the test value is 655.2988.
Embodiment 3
[0059] Embodiment 3: prepare aforementioned material C04
[0060] Using the same preparation method and raw material ratio as the preparation of material C01 in Example 1, except that diphenylamine is replaced by bis([1,1'-diphenyl]-4-yl)amine in step (4), The rest remain unchanged. The material C0 finally obtained is 99.45% through high-performance liquid chromatography (being called for short HPLC) detection purity, and learns through high-resolution mass spectrometry, theoretical value [M + ] is 723.2674 and the test value is 723.2675.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com