Isolated culture method for human endometrial tissue-derived endothelial progenitor cell
A technology of endothelial progenitor cells and endometrium, applied in the field of endothelial progenitor cells with strong angiogenesis ability, can solve the problems of limited angiogenesis ability and limited source of endothelial progenitor cells, and achieve the effect of strong angiogenesis ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
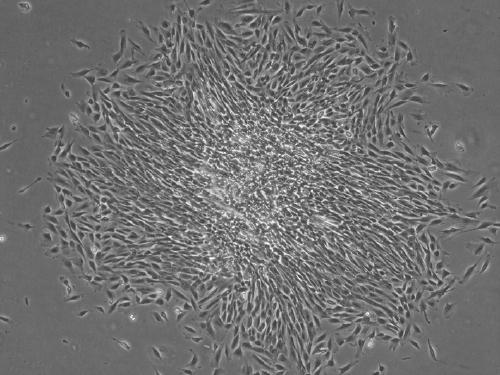
[0040] Example 1: Isolation and culture of human endometrium endothelial progenitor cells.
[0041] 1) Obtain human endometrial tissue, place it in sterile 1×PBS, and move it into the ultra-clean operating table as soon as possible.
[0042] 2) In a sterile culture vessel, the human endometrial tissue was repeatedly washed with PBS 3 times to remove surface blood and other impurities.
[0043] 3) Fully mince the cleaned human endometrial tissue to less than 1mm 3 .
[0044] 4) Add 3 times the volume of PBS digestion solution containing 300 µg / ml collagenase III and 40 µg / ml DNase I to the minced human endometrial tissue, shake and digest in a 37°C water bath at a shaking speed of 110rpm for 45min, and extract the digested product The supernatant was neutralized by adding 10% FBS-DMEM / F12 at a volume ratio of 1:1 to terminate the digestion.
[0045] 5) Add the above digestion solution to the undigested tissue fragments again, and repeat the digestion process of step 4).
[...
Embodiment 2
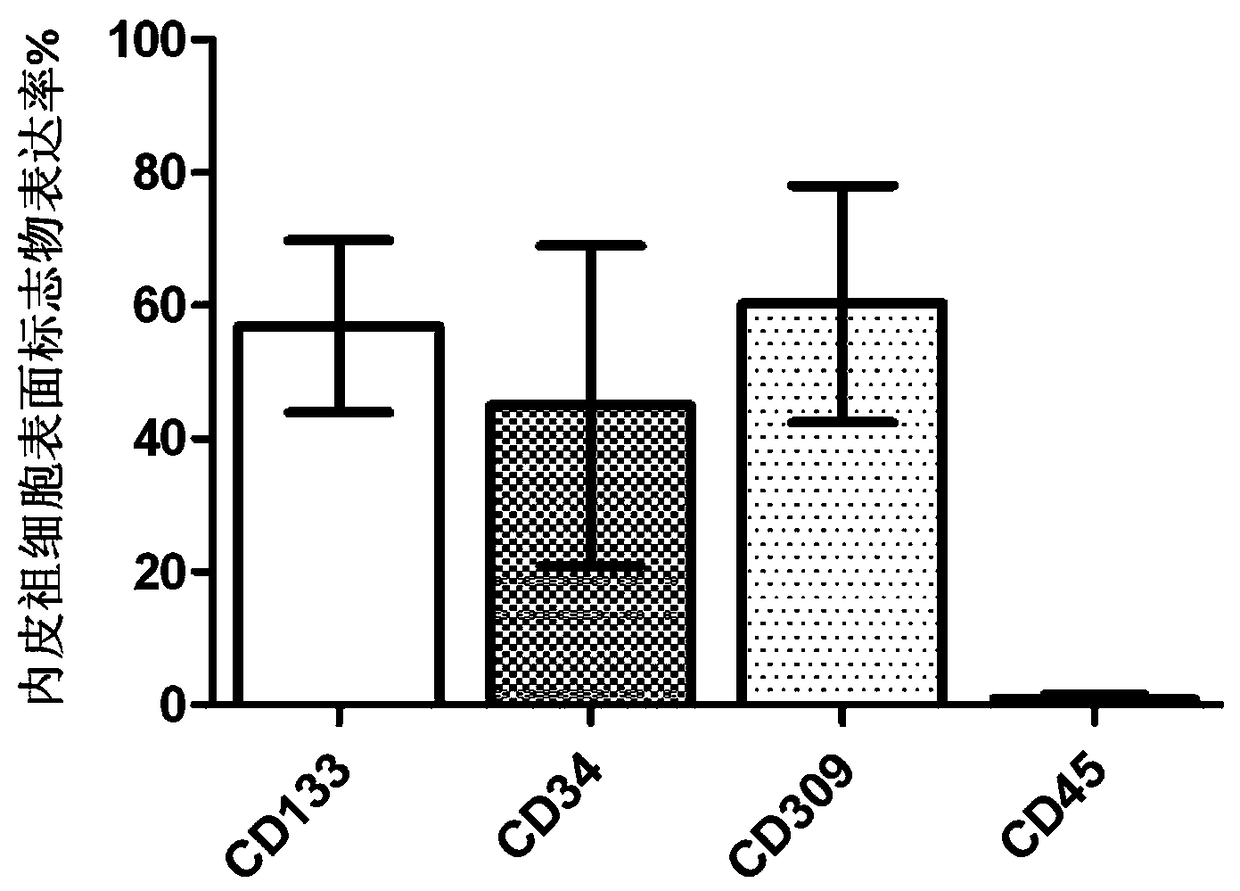
[0060] Example 2: Flow cytometric identification of human endometrial endothelial progenitor cells.
[0061] 1) The surface markers of human endometrium endothelial progenitor cells are CD133, CD34, CD309 and CD45.
[0062] 2) Select the endothelial progenitor cells cultured to the 7th day, 14th day, and 21st day respectively, and after routine digestion, stain with the antibody corresponding to the above-mentioned surface markers.
[0063] 3) Detection of surface markers of human endometrium endothelial progenitor cells by flow cytometry.
[0064] figure 2 The results of flow cytometry identification on the 14th day of culture are given, and the histogram shows the positive percentage of various surface markers.
[0065] figure 2 showed that the isolated and cultured human endometrial endothelial progenitor cells displayed CD133 + , CD34 + , CD309 + and CD45 - The characteristics of the surface markers are consistent with the characteristics of the surface markers of...
Embodiment 3
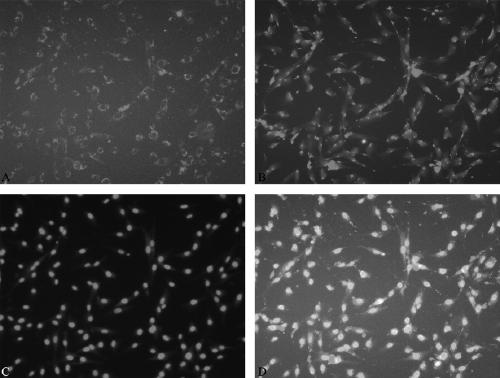
[0066] Example 3: Functional identification of human endometrium endothelial progenitor cells uptake Dil-ac-LDL and bind FITC-UEA-1.
[0067] 1) Sow human endometrial endothelial progenitor cells in a 6-well plate, place glass slides in advance in the 6-well plate, add EGM-2 culture medium, observe the growth of the cells, and wait until the cells are fused to 50-70%.
[0068] 2) Add 1ml of EGM-2 containing 10µg / ml Dil-ac-LDL into the culture well, and culture in the dark for 18h in a cell culture incubator at 37°C.
[0069] 3) After 18 hours, suck out the EGM-2 solution containing Dil-ac-LDL, wash 3 times with PBS, and fix with 4% paraformaldehyde for 30 minutes at room temperature.
[0070] 4) Wash with PBS 3 times.
[0071] 5) Add 1ml of 10µg / ml FITC-UEA-1 working solution and incubate at 37°C for 2h in the dark.
[0072] 6) After 2 hours, suck out the FITC-UEA-1 working solution and wash with PBS 3 times.
[0073] 7) Add 1ml of 3µg / ml DAPI and incubate at 37°C for 10min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com