Preparation method of novel electrophilic reagent-pyrimidine methanesulfonate
A technology of pyrimidine mesylate and hydroxypyrimidine, which is applied in the field of preparation of new electrophilic pyrimidinyl mesylate, can solve the problems that the synthesis method of new electrophile pyrimidinyl mesylate has not been reported in the literature, and achieves the goal of preparing Simple process, high yield, easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The invention provides a method for preparing pyrimidine mesylate represented by formula (1), the method comprising: using 2-hydroxypyrimidine and methanesulfonyl chloride represented by formula (2) as reaction raw materials, and using alkali as binding agent Acid agent, reacts in organic solvent, prepares pyrimidine mesylate,
[0021]
[0022] Among them, R 1 C1-C5 alkyl, R 2 is H, X, C1-C5 alkyl or C1-C5 alkoxy, and OMs is methylsulfonic acid group.
[0023] In a specific embodiment, R 1 is methyl (Me), ethyl, propyl, butyl or pentyl. In the preferred case, R 1 is methyl or ethyl.
[0024] In a specific embodiment, R 2 is H, F, Cl, Br, methyl, ethyl, propyl, butyl, pentyl, methoxy, ethoxy, propoxy, butoxy or pentyloxy. In the preferred case, R 2 for H, Cl, CH 3 or OCH 3 .
[0025] In a specific embodiment, the structural formula of pyrimidine mesylate shown in formula (1) is as follows:
[0026]
[0027]
[0028] In the method of the present inven...
Embodiment 1-8
[0038] Add 2.0mmol of 2-hydroxypyrimidine represented by formula (2), 4.0mmol of methanesulfonyl chloride and 10mL of dichloromethane into a 50mL round bottom flask, and slowly add 10.0mmol of Et 3 N, the temperature was raised to room temperature and stirred for 2 h, and TLC detected that the reaction was complete. Transfer the reaction solution into a separatory funnel, add 10mL of water, vibrate and stand to separate layers, collect the lower organic phase, evaporate the solvent under reduced pressure, and recrystallize the obtained crude product with ethanol to obtain pyrimidine mesylate.
[0039] Wherein, the corresponding group in the 2-hydroxypyrimidine shown in the formula (2) that adopts in each embodiment and the pyrimidine mesylate shown in the prepared formula (1) and the yield of target product pyrimidine mesylate As shown in Table 1 below.
[0040] Table 1
[0041]
[0042]
[0043] The structural formulas of the target products prepared in Examples 1-8 a...
Embodiment 1
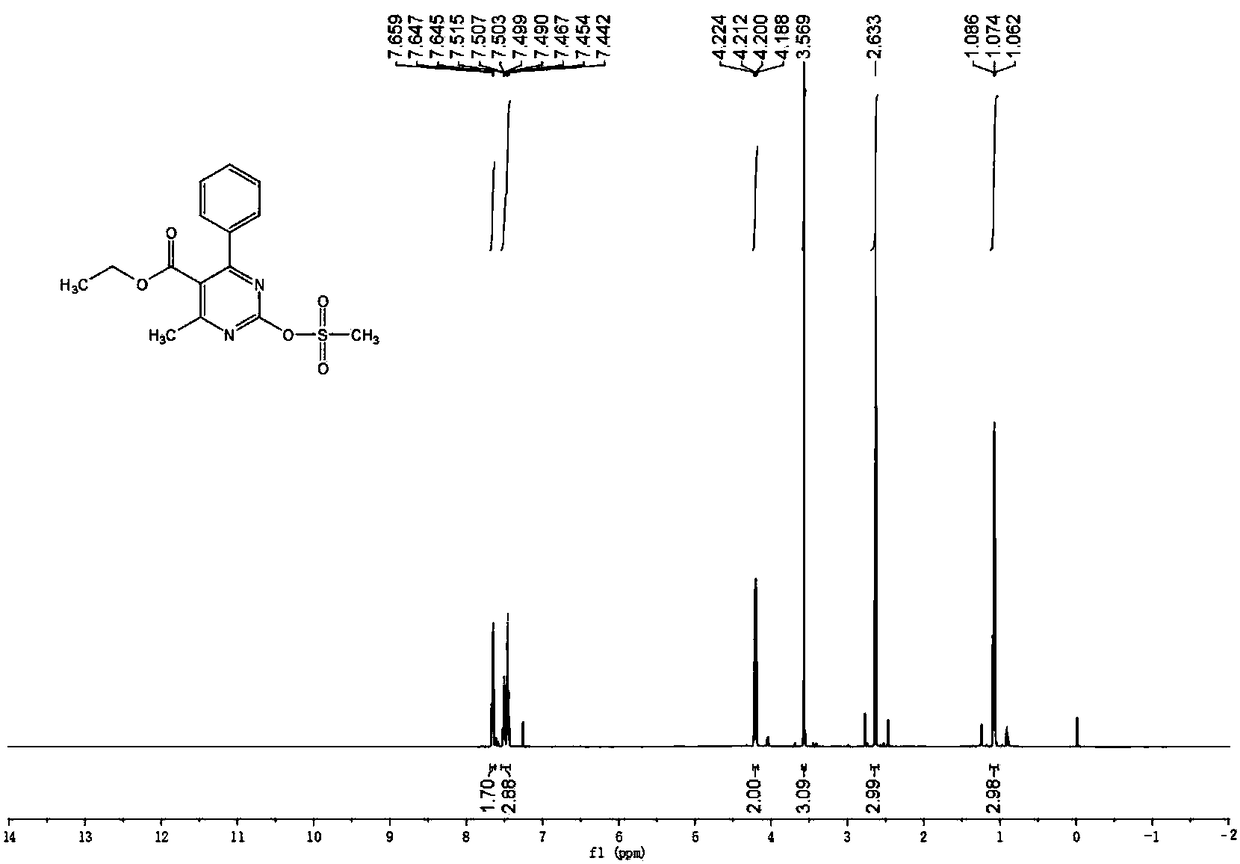
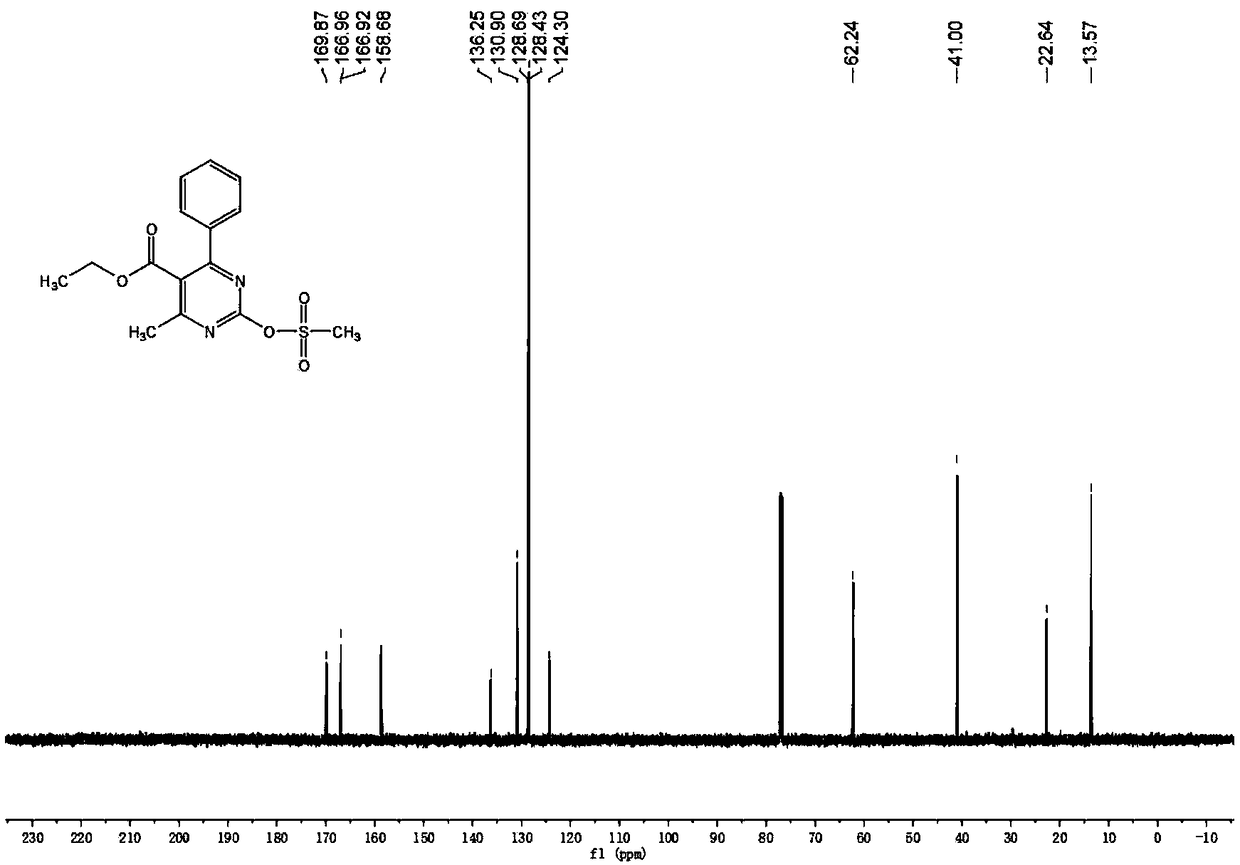
[0048] The characterization data of the target product prepared in Example 1 are as follows: colorless crystals, mp: 96-98°C, yield: 87.2%; 1 H NMR (600MHz, CDCl 3 ):δ7.65(d,J=7.2Hz,2H),7.52-7.44(m,3H),4.21(q,J=7.2Hz,2H),3.57(s,3H),2.63(s,3H) ,1.07(t,J=7.2Hz,3H). 13 C NMR (150MHz, CDCl 3 ): δ169.87, 166.96, 166.92, 158.68, 136.25, 130.90, 128.69, 128.43, 124.30, 62.24, 41.00, 22.64, 13.57.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com