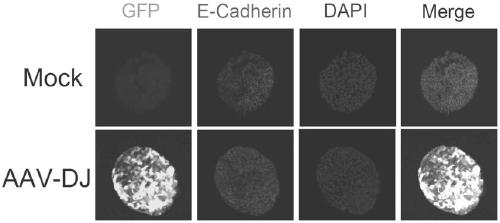
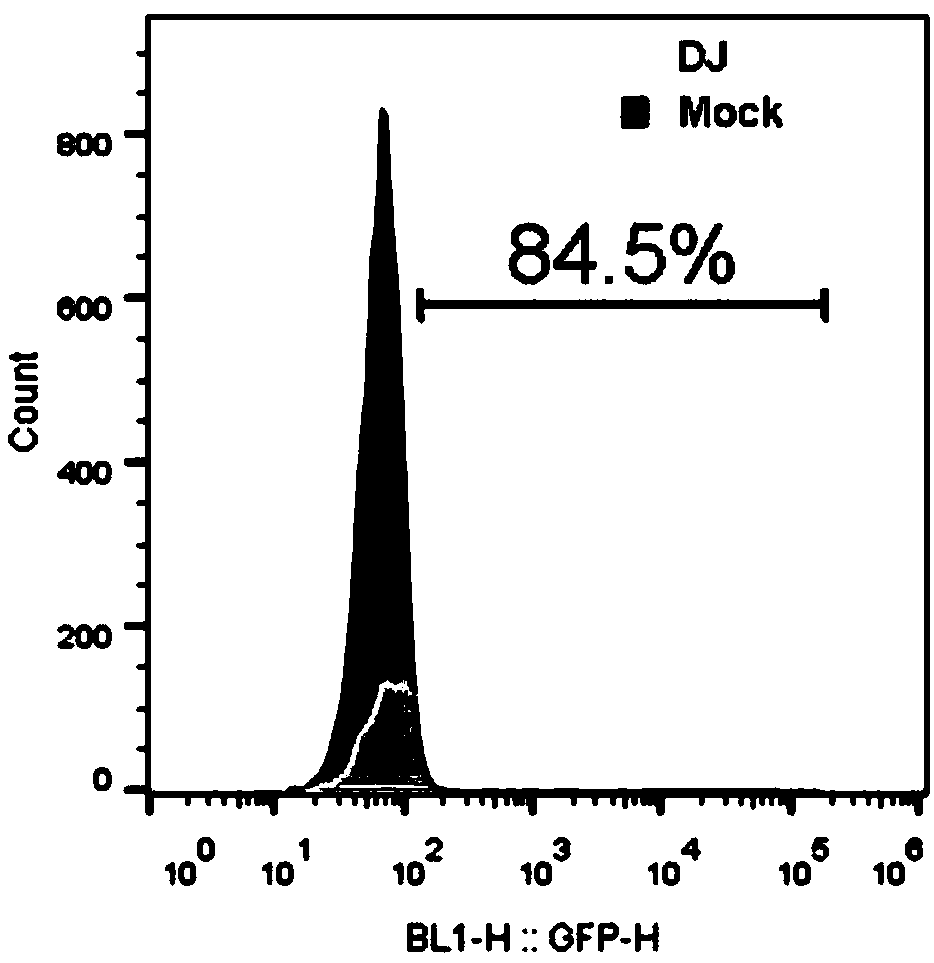
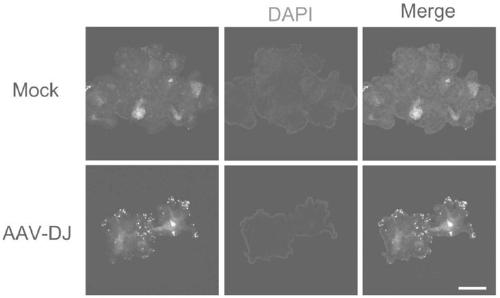
Usage of AAV-DJ type adeno-associated virus in-vitro efficient infection organoid
An AAV-DJ, organoid technology, applied in the field of genetic engineering and cell engineering, can solve the problem of less research and achieve the effect of efficient infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Isolation and culture of liver organoids
[0032] For the isolation and culture of liver organoids, refer to the method reported in [Broutier, et al. (2016). Culture and establishment of self-renewing human and mouse adult liver and pancreas 3 Dorganoids and their genetic manipulation. Nature protocols 11, 1724-1743.] , Liver organoids were isolated from liver tissues of wild-type C57 / B6 mice and humans for virus infection. The specific methods are as follows:
[0033] 1. Immediately after the mouse was euthanized, the liver was taken out, and placed in the 4-degree pre-cooled basal medium, the basal medium components were: Advanced DMEM / F12 medium, penicillin, GlutaMax and HEPES;
[0034] 2. Cut the liver to 0.5mm with surgical scissors 3 Transfer it to a 15ml centrifuge tube, add 10ml washing medium (composition: DMEM medium, 1% fetal bovine serum, 1% penicillin) and beat repeatedly;
[0035] 3. Allow to settle and remove the supernatant. Then add 10ml o...
Embodiment 2
[0044] Example 2: Isolation and culture of intestinal organoids
[0045] Isolation and culture of small intestine organoids refer to the method reported in [Sato, et al. (2009). Single Lgr5 stemcells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262-265.], from wild-type Small intestinal organoids were isolated from C57 / B6 mouse and human liver tissues for virus infection. The specific method is as follows:
[0046] 1. After the mice were euthanized, the small intestines were taken out and placed in 4-degree pre-cooled PBS buffer;
[0047] 2. Cut the small intestine longitudinally with surgical scissors, and rinse with PBS buffer 3 times to remove chyme;
[0048] 3. Use a scalpel to carefully scrape off the small intestinal villi on the inner wall of the small intestine;
[0049] 4. Cut the remaining part into 5mm length intestinal slices and incubate on ice with PBS buffer containing 10mM EDTA for 40 minutes;
[0050] 5. Replace the PBS bu...
Embodiment 3
[0053] Example 3: AAV-DJ virus infects organoids (Here, liver organoids are taken as an example, small intestine and kidney organoids are operated similarly)
[0054] 1. Separate the organoids in 3D culture together with Matrigel from the well plate, rinse the well plate once with PBS, and collect it in a 1.5ml centrifuge tube;
[0055] 2. Centrifuge at 900rpm for 1min and remove the supernatant;
[0056] 3. Add 1ml of PBS buffer solution, blow and mix well to remove the matrigel around the organoids;
[0057] 4. Centrifuge at 1000 rpm for 1 min and remove the supernatant;
[0058] 5. Spread 40 μl of pre-cooled Matrigel evenly in a 24-well plate, and let it solidify for 10 minutes;
[0059] 6. Dilute AAV-DJ virus with 400 μl organoid medium, according to MOI=10E6 (the higher the MOI, the higher the infection efficiency), that is, to infect 10,000 cells, only 1 μl AAV-DJ virus (titer is 10E13 vgs / ml);
[0060] 7. Resuspend the organoid pellet in step 4 with the virus-added ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com