Preparation method of methyl 5-formyl-2-methoxybenzoate
A technology of methyl methoxybenzoate and methyl methoxybenzoate, applied in the field of medicine, can solve problems such as low yield of methyl 5-formyl-2-methoxybenzoate, and achieve easy operation and control, short reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
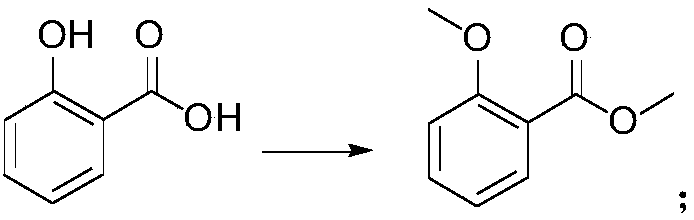
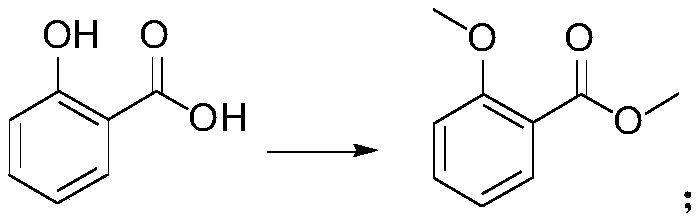
[0034] (1) the preparation of 2-methoxymethyl benzoate, reaction formula is as follows:
[0035]
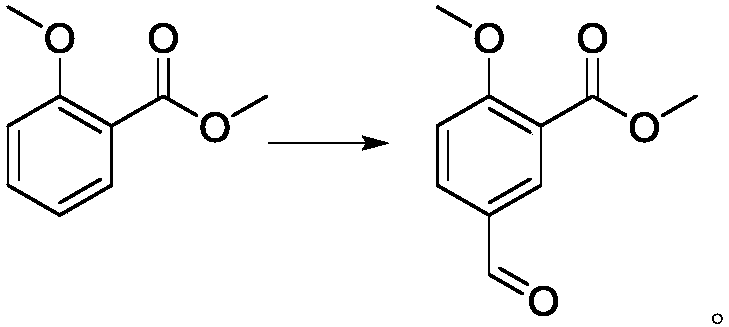
[0036] (2) the preparation of 5-formyl-2-methoxybenzoic acid methyl ester, reaction formula is as follows:
[0037]
Embodiment 1
[0039] (1) Preparation of methyl 2-methoxybenzoate: add 150kg salicylic acid, 500L acetone, 225kg potassium carbonate to the reaction flask, heat up to 50-60°C, add 274kg dimethyl sulfate dropwise, and react for 3 hours , added 150kg potassium carbonate, 137kg dimethyl sulfate, reacted for 24 hours, after the reaction was completed, 300L of solvent was evaporated, cooled to 20°C, 500L of water was added, filtered, the filtrate was extracted with 500LDCM, and concentrated to obtain the product, 178.6kg, yield : 99%
[0040] (2) Preparation of methyl 5-formyl-2-methoxybenzoate: cool methyl 2-methoxybenzoate (100kg) and methanesulfonic acid (300L) to 0-10°C, add Hexatropine (252kg), heat up to 90°C, react for 16 hours, after the reaction is complete, cool down to room temperature, add 500L water into the reaction bottle, adjust ph=6-7 with sodium hydroxide solution, filter, and rinse the filter cake with water Wash and dry to obtain product, 109.8kg, yield 94%.
[0041] 1H NMR:...
Embodiment 2
[0043] (1) Preparation of methyl 2-methoxybenzoate: add 150kg salicylic acid, 500L acetone, 375kg potassium carbonate to the reaction flask, heat up to 50-60°C, slowly add 410kg dimethyl sulfate dropwise for 24 hours After dripping, keep stirring and react for 24 hours. After the reaction is completed, evaporate the solvent, cool down to 20°C, add 550L of water, filter, extract the filtrate with 500L of DCM, and concentrate to obtain the product, 175.3kg, yield: 97%
[0044] (2) Preparation of methyl 5-formyl-2-methoxybenzoate: cool methyl 2-methoxybenzoate (100kg) and methanesulfonic acid (400L) to 0-10°C, add Hexatropine (252kg), heat up to 80°C, react for 16 hours, after the reaction is complete, cool down to room temperature, add 500L water into the reaction bottle, adjust ph=6~7 with sodium hydroxide solution, filter, and rinse the filter cake with water Wash and dry to obtain the product, 99.3kg, yield 85.1%.
[0045] 1H NMR: (DMSO-d6,300MHz)6 9.92(5,1H),8.20-8.21(d,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com