Automatic verification method for clinical test source data
A technology for clinical trials and automatic verification, applied in the field of data verification, can solve problems such as inability to guarantee quality, lack of energy data, verification, etc., to achieve the effect of improving service capabilities and intelligence levels, ensuring data quality, and reducing workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The present invention will be described in further detail below in conjunction with the accompanying drawings and specific embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
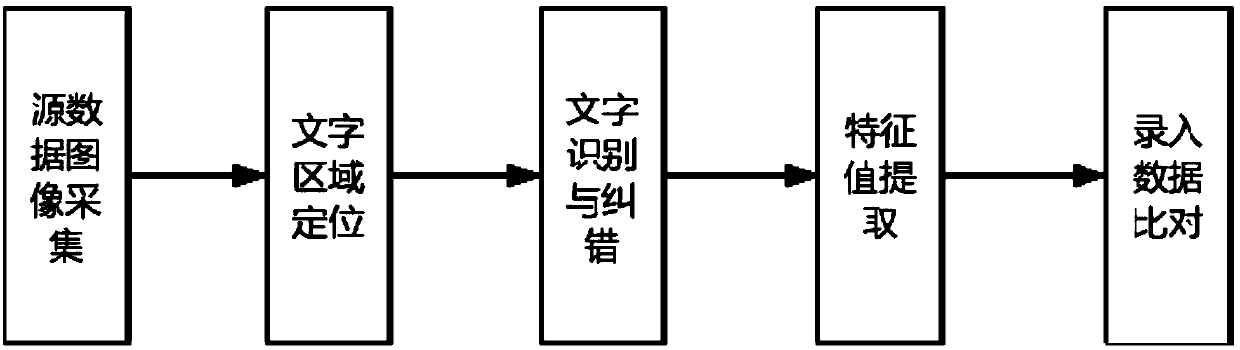
[0027] The present invention includes five parts: source data image acquisition, text area positioning, text recognition, feature value extraction and input data comparison. Firstly, target is determined through text area positioning, text content is extracted by text recognition algorithm, and then clinical trial data is obtained. The characteristic values that are concerned in the process, and finally compare the results of the automatic extraction of characteristic values with the characteristic values entered by the investigators, and identify and warn the wrong entries.
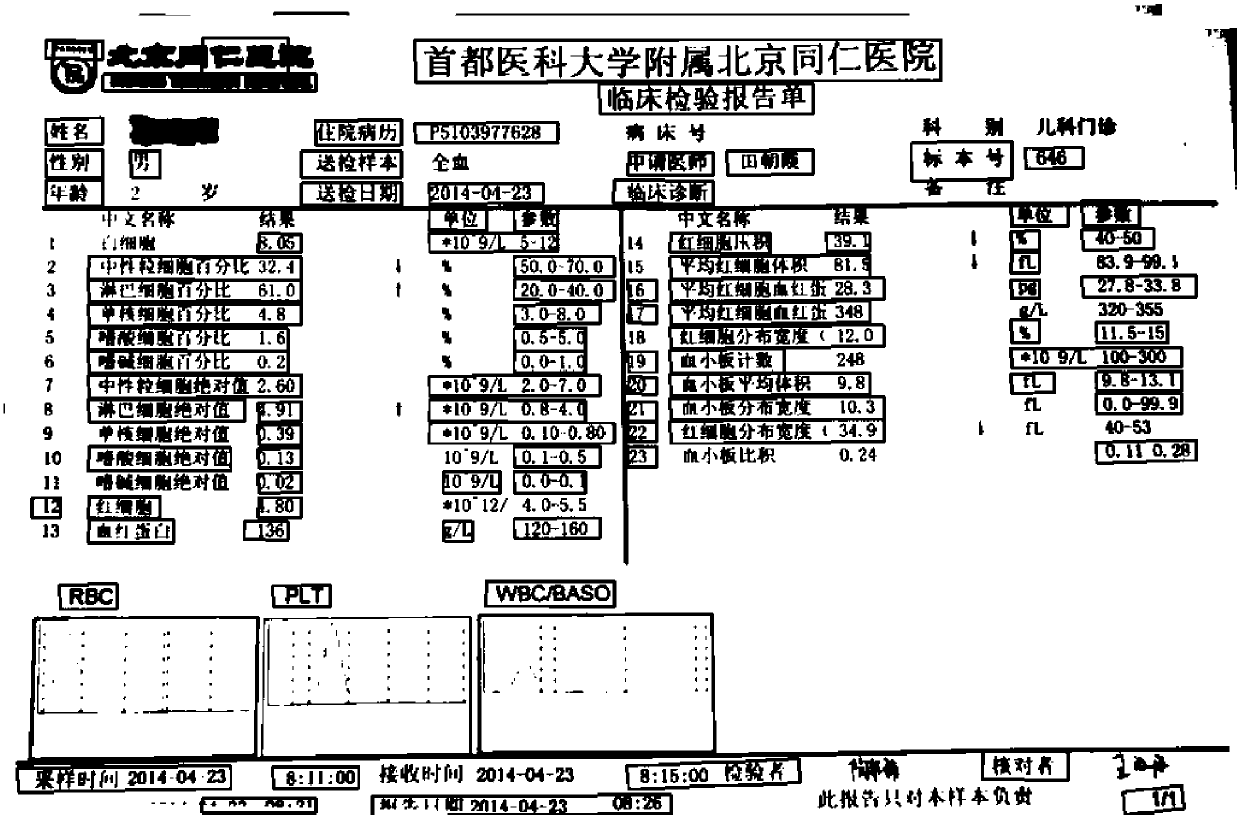
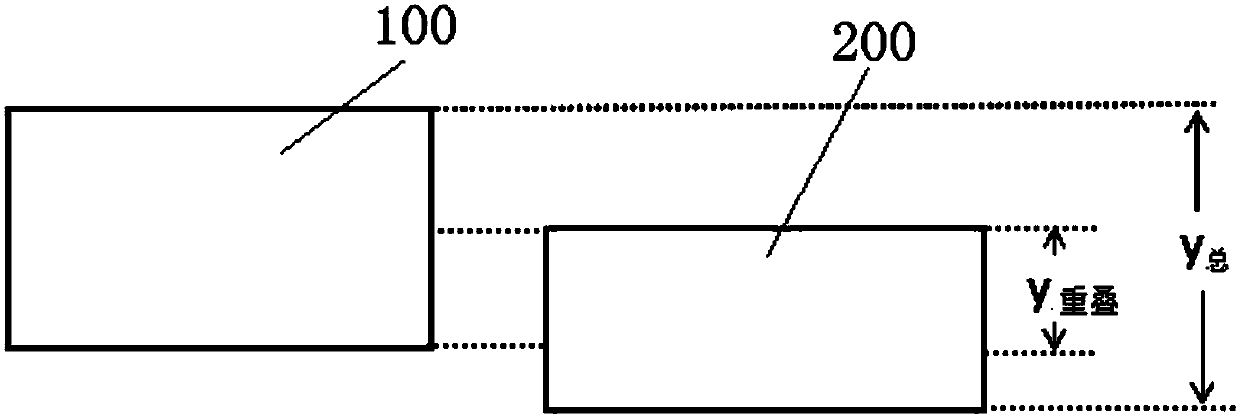
[0028] see Figure 1-5 As shown, an automatic verification method for clinical trial source data, incl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com