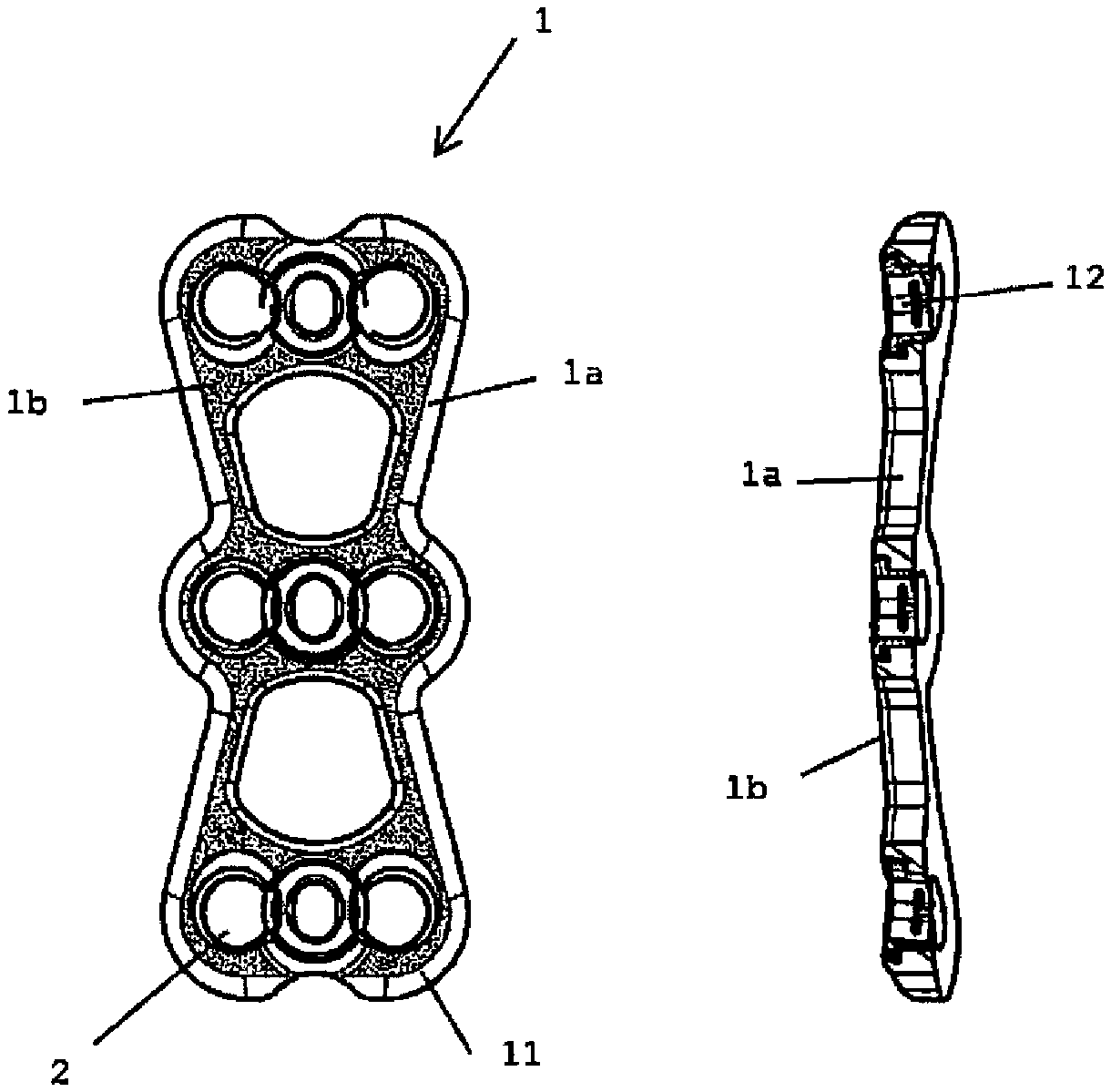
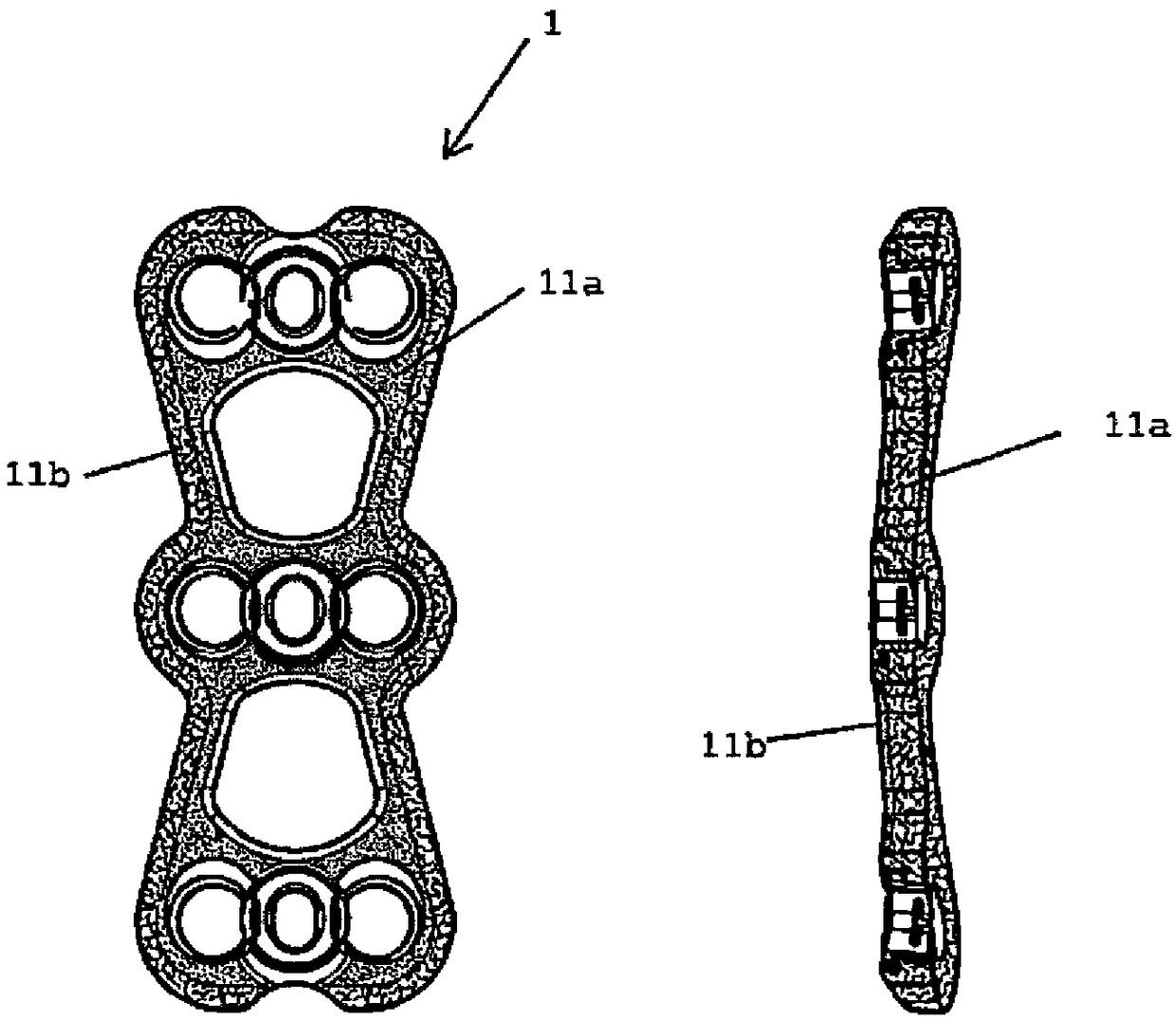
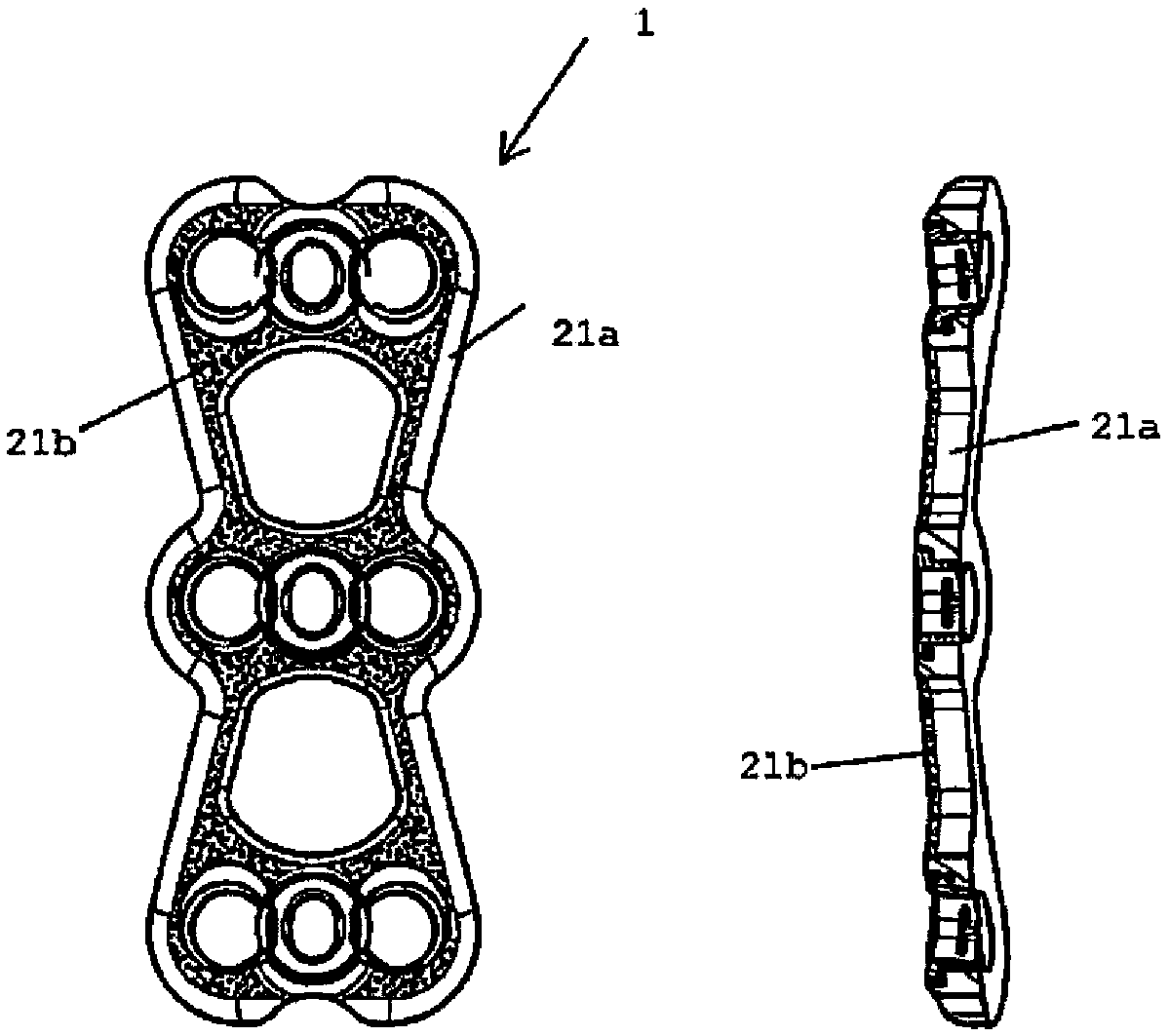
Antibacterial device to be implanted in vivo
A technology for implanting devices and implants, applied in prosthesis, internal bone synthesis, medical science, etc., can solve the problem of difficulty in preventing the growth of microorganisms, and achieve the effect of reducing the burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0058] (Preparation of samples)
[0059] Commercially available biocompatible titanium alloy (titanium content: 88 to 91 mass%) and commercially available biocompatible cobalt-chromium alloy (cobalt content: 58 to 69 mass%, chromium content: 26 to 30 mass%) 3 mm thick coin-shaped samples were processed into various areas shown in Table 1 (only the titanium alloy of sample No. short circuit. As comparative samples, a flat polyethylene sample of 1 mm thickness (control), a coin-shaped sample of 3 mm thickness made of cobalt-chromium alloy (comparison 1), and a coin-shaped sample of 3 mm thickness made of titanium alloy were similarly prepared (compare 2).
[0060] [Table 1]
[0061] Table 1 Sample Conditions
[0062]
[0063]
[0064] *Area ratio = surface area of titanium alloy / surface area of cobalt-chromium alloy
[0065] (antimicrobial properties test)
[0066] Antimicrobial properties are evaluated with reference to JIS Z 2801: Antimicrobial Products - Antim...
example 2
[0075] (Preparation of samples)
[0076] An implant device made of a titanium alloy and a cobalt-chromium alloy (the area ratio of the titanium alloy to the cobalt-chromium alloy was set to 0.89) was prepared (implant device 1). As a comparative example, an implant device (implant device 2 ) made only of a titanium alloy having exactly the same shape was similarly prepared.
[0077] [table 3]
[0078] Table 3 Composition of the implanted device
[0079]
[0080] *Area ratio = surface area of member made of titanium alloy / surface area of member made of cobalt-chromium alloy
[0081] (antimicrobial properties test)
[0082] Each implant was placed in a polypropylene tube and immersed in 1 ml of Staphylococcus aureus (NBRC 12732) suspended in nutrient broth (1 / 100). After culturing at 35°C for 24 hours, the medium was collected. The collected medium was serially diluted in 10-fold dilution series, and cultured at 35° C. for 24 hours using an agar plate culture method,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com