PEGylated bioactive peptides and uses thereof
A technology of drugs and analogues, applied in the field of PEGylated bioactive peptides and their uses, can solve problems such as inactivity and low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0201] Example 1: Materials and methods
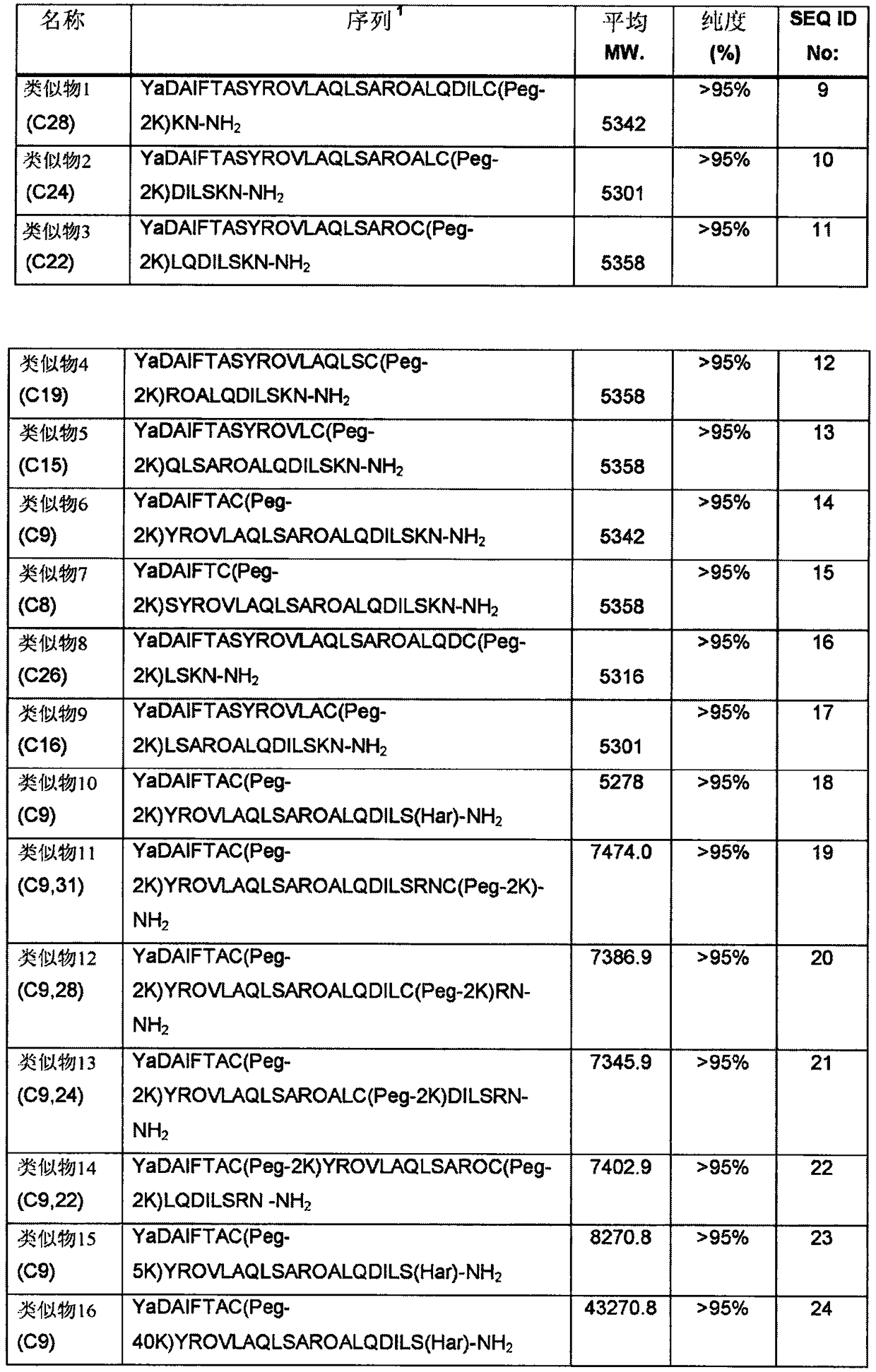
[0202] Synthesis and Preparation of GHRH Analogs
[0203] Using fluorenylmethoxycarbonyl-protected α-amino acids with appropriate side chain protection and benzhydrylamine (BHA) resin (Bachem AG) at a loading of 0.75 mmol / g, using manual or automated solid-phase peptide synthesis methods Preparation of GHRH analogs described herein. Prior to amino acid coupling, 6-aminocaproic acid and Rink linkers were coupled to the resin, followed by [2-(6-chloro-1H-benzotriazol-1-yl)-1,1,3,3 -tetramethylammonium hexafluorophosphate] (HCTU) or (1-cyano-2-ethoxy-2-oxoethyleneaminooxy)dimethylamino-morpholino-carbon Coupling of hexafluorophosphate (COMU) and diisopropylethylamine (DIEA) in N,N-dimethylformamide (DMF) to Fmoc-[9H-fluoren-9-ylmethoxycarbonyl] protected Amino acid about 1 hour. Fmoc deprotection was performed using 20% (v / v) piperidine in DMF for about 0.5 hours. A general procedure for N-capping the peptides described herein wit...
Embodiment 2
[0207] Example 2: PEGylated GHRH Peptides in Cell-Based Hit (DiscoveRx TM ) Determination of Agonist Potency in Assay
[0208] DiscoverRx TM A panel of cell lines stably expressing an untagged GPCR that signals through cAMP was developed. Hit cAMP assay using DiscoverRx TM A technique called Enzyme Fragment Complementation (EFC), which has β-galactosidase (β-Gal) as a functional reporter, was developed to signal via Gs second messenger in a uniform non-imaging assay format Activation of GPCRs is monitored.
[0209] Amplify cAMP Hunter from frozen stocks according to standard protocols TM cell line. Cells were seeded into white-walled 384-well microplates in a total volume of 20 μL and incubated at 37°C for an appropriate time prior to testing. Using DiscoverRx TM Hit Hunter TM The cAMP XS+ assay determines cAMP regulation. For agonist assays, cells are incubated with samples to induce a response. Media was aspirated from the cells and replaced with 15 μL of 2:1...
Embodiment 3
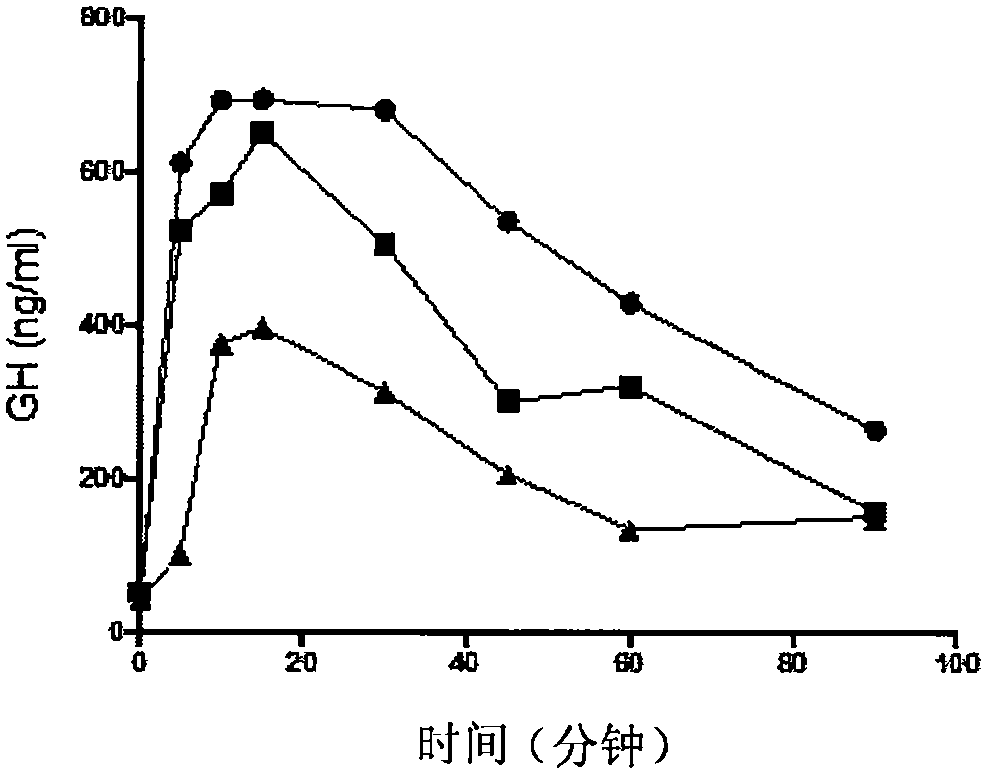
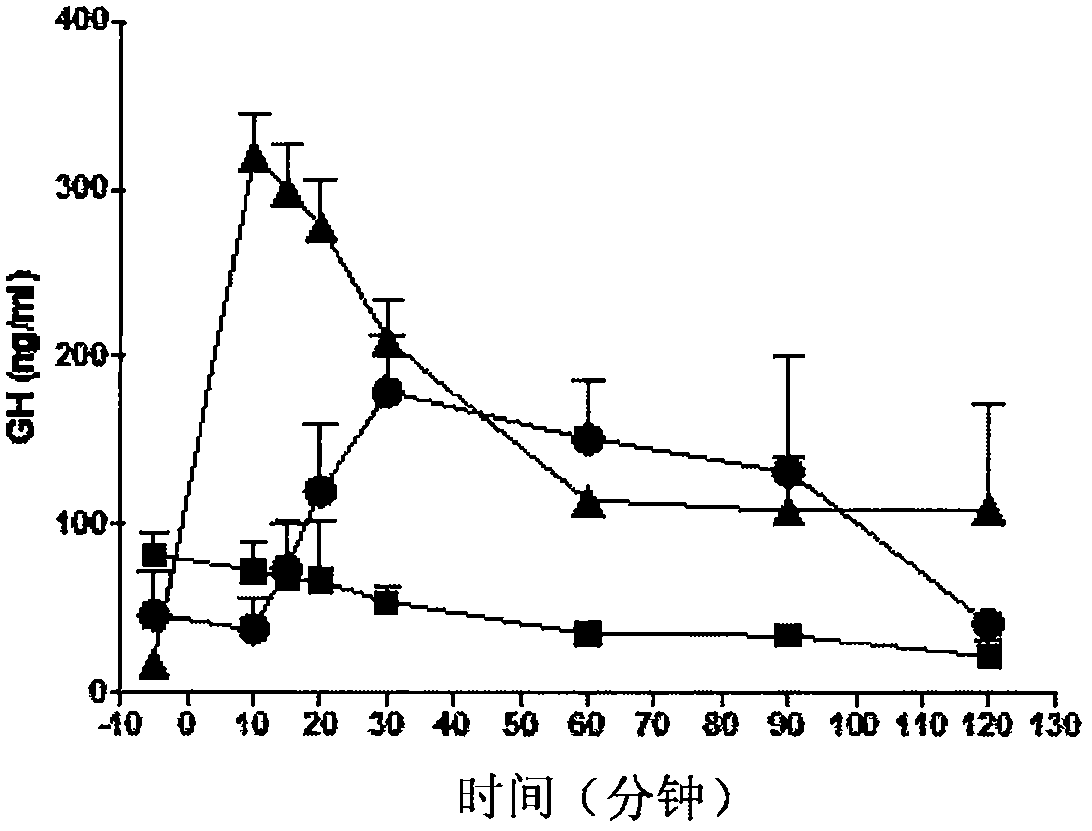
[0218] Example 3: GH Release Kinetics in Sprague-Dawley Rats in Response to Subcutaneous Injection of Selected GHRH Analogs
[0219]Protocol: Sprague-Dawley rats (female, body weight 250 to 300 g) were obtained from Charles River Inc. Animals were used according to the protocols of the Committee on Animal Care and the principles of the Canadian Council for Animal Care's Guide for the Care and Use of Laboratory Animals. Animals were maintained on standard laboratory chow on a 12:12 light:dark cycle. They were maintained in groups of 4 rats per cage. Anesthetize the animal with isoflurane 2.5%. A midsection opening is made in the neck to expose the carotid artery. The carotid artery was cannulated with polypropylene tubing (PE-50) to allow blood draw. Following surgical preparation, rats received subcutaneous injections of GHRH analogs (10 to 100 μg / kg) dissolved in 20 mM AcONa + 5% mannitol (pH 5). Blood samples (400 μL / time point) were collected from 2 to 4 animals per gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com