Poly-2,5-butanediol furadicarboxylic acid copolyester and preparation method thereof
A technology of furan butanediol copolyester and furandicarboxylic acid, which is applied in the field of materials and polymers, can solve the problems of brittleness, poor impact strength, easy crystallization, etc., and achieves good toughness, elongation at break and impact resistance. high intensity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention provides a kind of preparation method of the copolyester of formula (I) structure, comprising:
[0031] A) 2,5-furandicarboxylic acid or its derivatives are mixed with 1,4-butanediol and a long-chain diol of formula (II) and a catalyst to obtain a prepolymer; the 2,5-furandicarboxylic acid or its derivatives are 2,5-furandicarboxylic acid or dimethyl 2,5-furandicarboxylate;
[0032] B) the prepolymer is carried out polycondensation reaction, obtains the poly-2,5-furandiol copolyester of formula (I) structure;
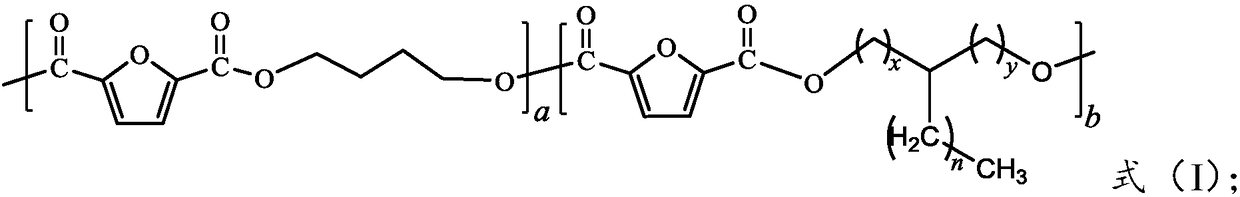
[0033]
[0034] Where a≧1, b≧1, n≧1, x≧0, y≧0, and x and y are both 0.
[0035] In the present invention, first, 2,5-furandicarboxylic acid or its derivatives, 1,4-butanediol, long-chain dihydric alcohols with the structure of formula (II), and a catalyst are added into a reaction flask, and stirred and reacted under the protection of an inert gas to obtain prepolymer.
[0036] According to the present invention, the molar ratio of the...
Embodiment 1
[0054] Add 1.00mol 2,5-furandicarboxylic acid, 1.52mol 1,4-butanediol and 0.08mol 1,2-decanediol into the reaction flask, stannous oxalate is used as catalyst, accounting for 0.3 mole fraction of 2,5-furandicarboxylic acid %, under the protection of nitrogen, stirred and reacted at 230°C for 2h to form a prepolymer;
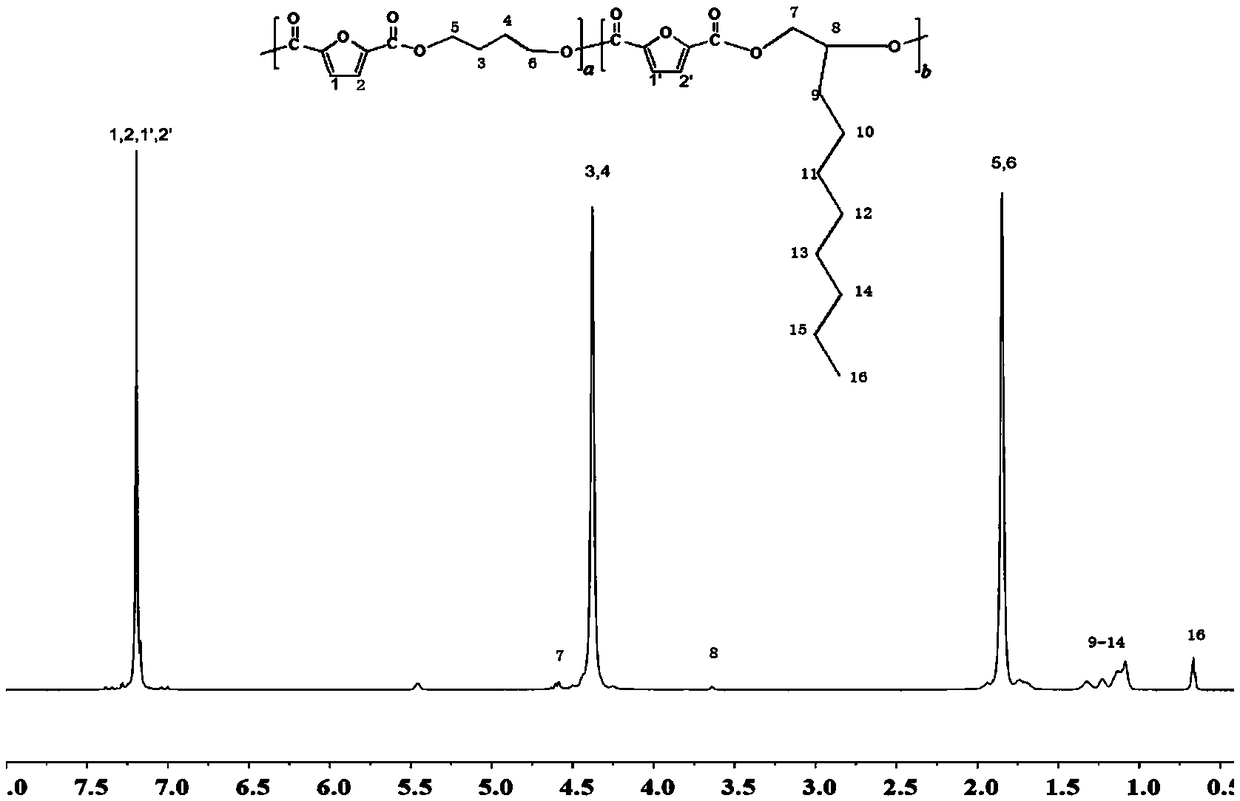
[0055] Vacuumize the above prepolymer to 0.070MPa, and stir at 240°C for 6h to obtain poly-2,5-furandicarboxylic acid-butylene glycol-1,2-decanediol copolyester (PBSF). Polybutylene 2,5-furandicarboxylate (PBF) synthesized under the same conditions was used as a comparison.
[0056] The performance of the poly-2,5-furandicarboxylic acid-butylene glycol-1,2-decanediol copolyester prepared in Example 1 of the present invention was measured, and at the same time, the poly-2,5-furandiol synthesized under the same conditions Butylene glycol formate (PBF) was used for comparative determination. The result is as figure 1 and shown in Table 1. in figure 1 The NMR fi...
Embodiment 2
[0060] Add 1.00mol of 2,5-furandicarboxylic acid, 1.52mol of 1,4-butanediol and 0.08mol of 1,2-dodecanediol into the reaction flask, tetrabutyl titanate is used as a catalyst, accounting for 2,5-furandicarboxylic acid moles 0.1% of the fraction, under the protection of nitrogen, stirred and reacted at 230°C for 1.5h to form a prepolymer;
[0061] The above prepolymer was evacuated to 0.070MPa, stirred and reacted at 240° C. for 6 hours to obtain poly-2,5-furandicarboxylic acid-butylene glycol-1,2-dodecanediol copolyester (PBTF).
[0062] The poly-2,5-furandicarboxylic acid-butylene glycol-1,2-dodecanediol copolyester prepared in Example 2 of the present invention was tested for performance, and at the same time, the poly-2,5-furan synthesized under the same conditions Butylene glycol diformate (PBF) was used for comparative determination. The results are shown in Table 2.
[0063] Table 2
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com