Secondary/tertiary amide compound and synthesis method thereof
A technology of amine compounds and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve problems such as harsh reaction conditions, and achieve the effects of direct reaction, high yield, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Preparation of N-(p-tolyl)benzamide
[0069] 0.75mmol of trimethoxyphenylsilane, 10mol%Pd(PPh 3 ) Cl 2 and 2equiv.CuF 2 dissolved in 3mLCH 3 In the Shrek tube of CN (equipped with a magnetic stirrer), close the reaction tube, pass carbon monoxide from the branch of the reaction tube, make it full of balloons and then empty them, repeat three times until the air in them is completely emptied, and then put Fill the balloon, then add 0.5mmol p-methylaniline into the reaction tube, heat and stir at 80°C for 24 hours, monitor the progress of the reaction with TLC, extract three times with 10mL ethyl acetate after the reaction is complete, combine the organic phases and concentrate , separated by silica gel column chromatography to obtain 97.1 mg of white solid compound, the yield rate is 92%, and the product structure formula is as follows:
[0070]
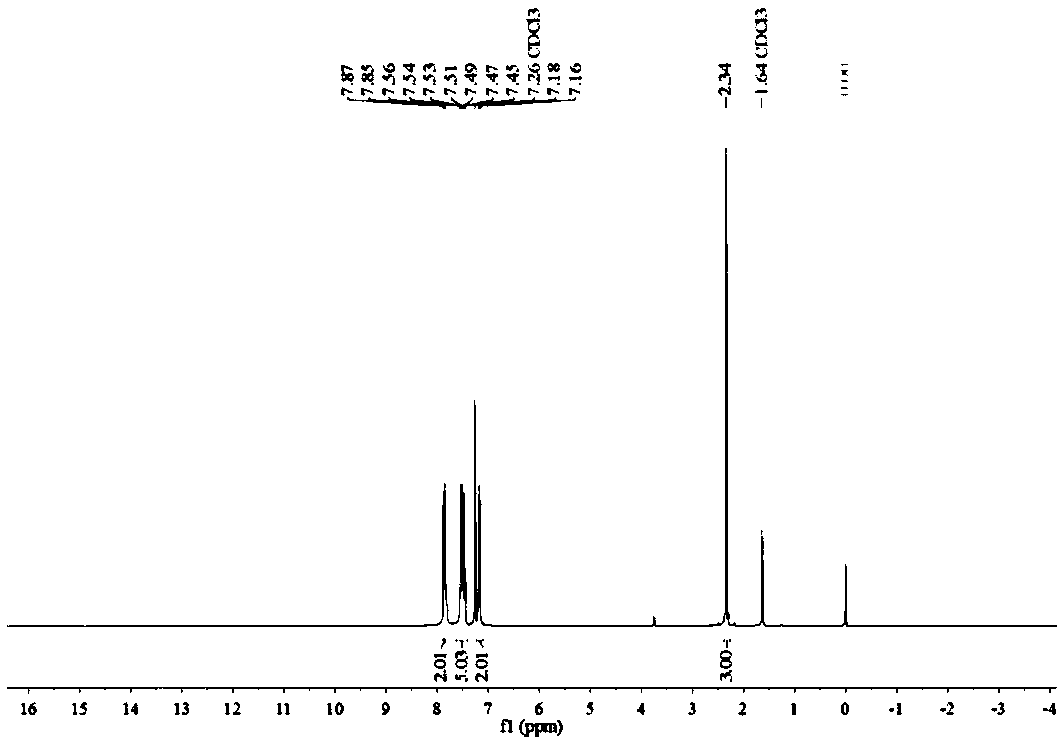
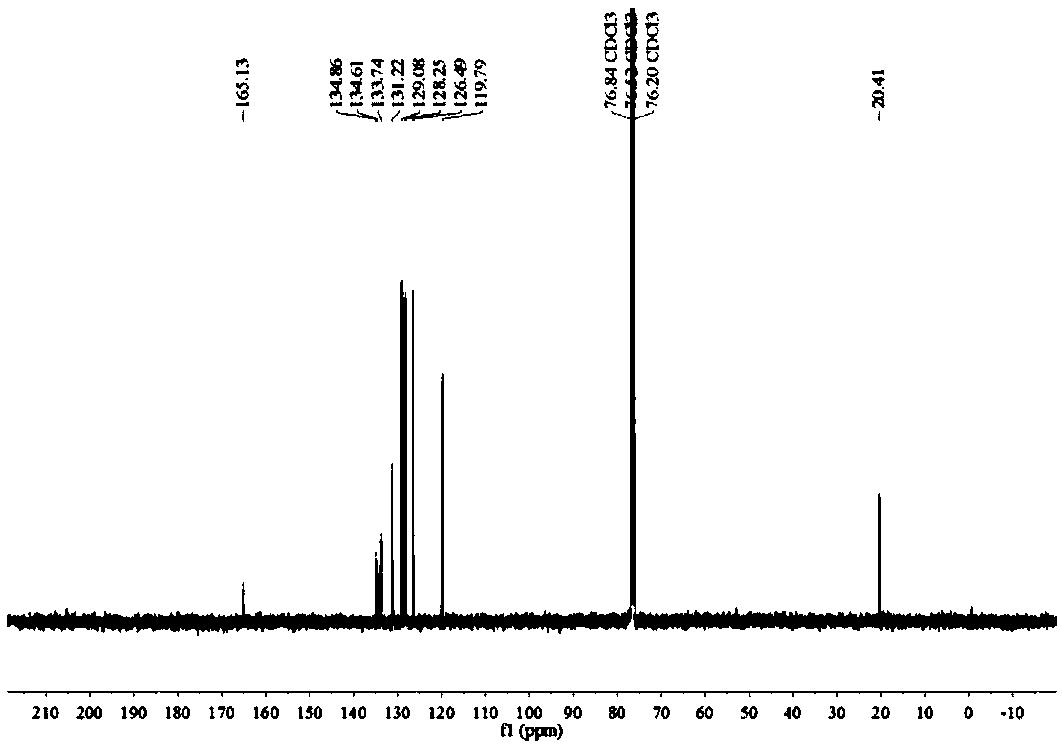
[0071] Such as figure 1 with figure 2 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 )δ7.86(d, J=7....
Embodiment 2
[0074] Preparation of N-(4-methoxyphenyl)benzamide
[0075] 0.75mmol of trimethoxyphenylsilane, 10mol%Pd(PPh 3 ) Cl 2 and 2equiv.CuF 2 dissolved in 3mLCH 3 In the Shrek tube of CN (equipped with a magnetic stirrer), close the reaction tube, pass carbon monoxide from the branch of the reaction tube, make it full of balloons and then empty them, repeat three times until the air in them is completely emptied, and then put Fill the balloon, then add 0.5mmol of p-methoxyaniline into the reaction tube, heat and stir at 80°C for 24 hours, monitor the progress of the reaction with TLC, extract three times with 10mL ethyl acetate after the reaction is complete, combine the organic phases and carry out Concentrate and separate through silica gel column chromatography to obtain 94.3 mg of white solid compound with a yield of 83%. The structural formula of the product obtained is as follows:
[0076]
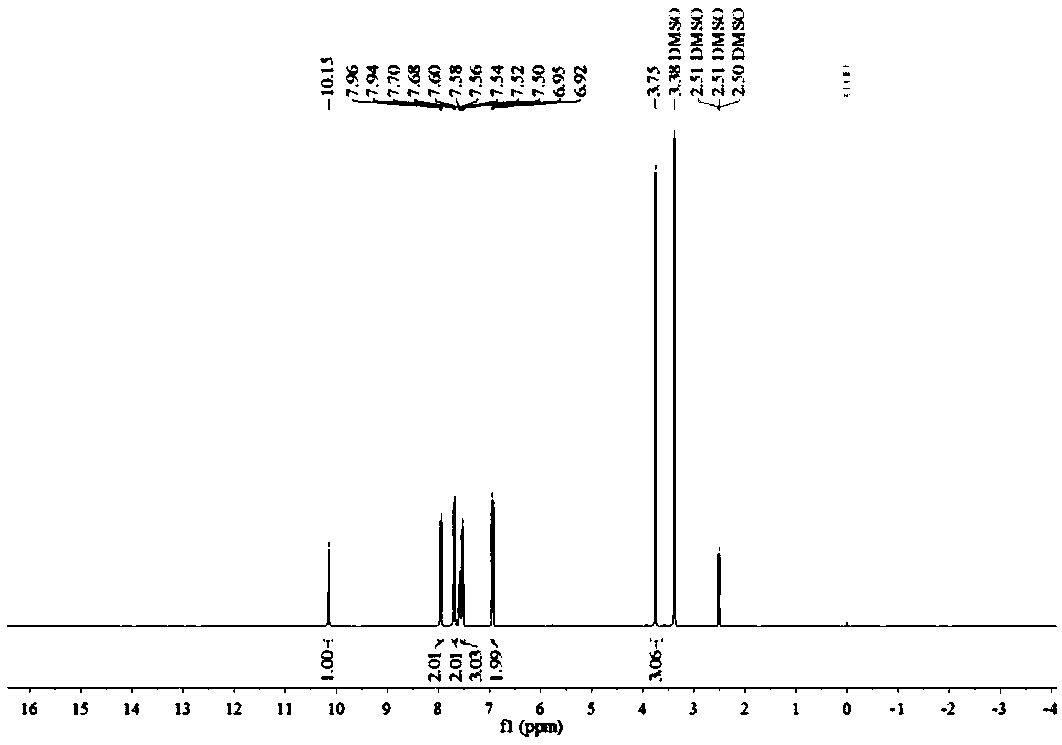
[0077] Such as image 3 with Figure 4 Shown, product NMR characterization: 1...
Embodiment 3
[0080] Preparation of N-(4-fluorophenyl)benzamide
[0081] 0.75mmol of trimethoxyphenylsilane, 10mol%Pd(PPh 3 ) Cl 2 and 2equiv.CuF 2 dissolved in 3mLCH 3 In the Shrek tube of CN (equipped with a magnetic stirrer), close the reaction tube, pass carbon monoxide from the branch of the reaction tube, make it full of balloons and then empty them, repeat three times until the air in them is completely emptied, and then put The balloon was filled, and then 0.5 mmol of p-fluoroaniline was added to the reaction tube, heated and stirred at 80°C for 24 hours, and the progress of the reaction was monitored by TLC. After the reaction was complete, it was extracted three times with 10 mL of ethyl acetate, and the combined organic phases were concentrated. After separation by silica gel column chromatography, 87.1 mg of white solid compound was obtained, with a yield of 81%, and the structural formula of the product obtained was as follows:
[0082]
[0083] Such as Figure 5 with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com