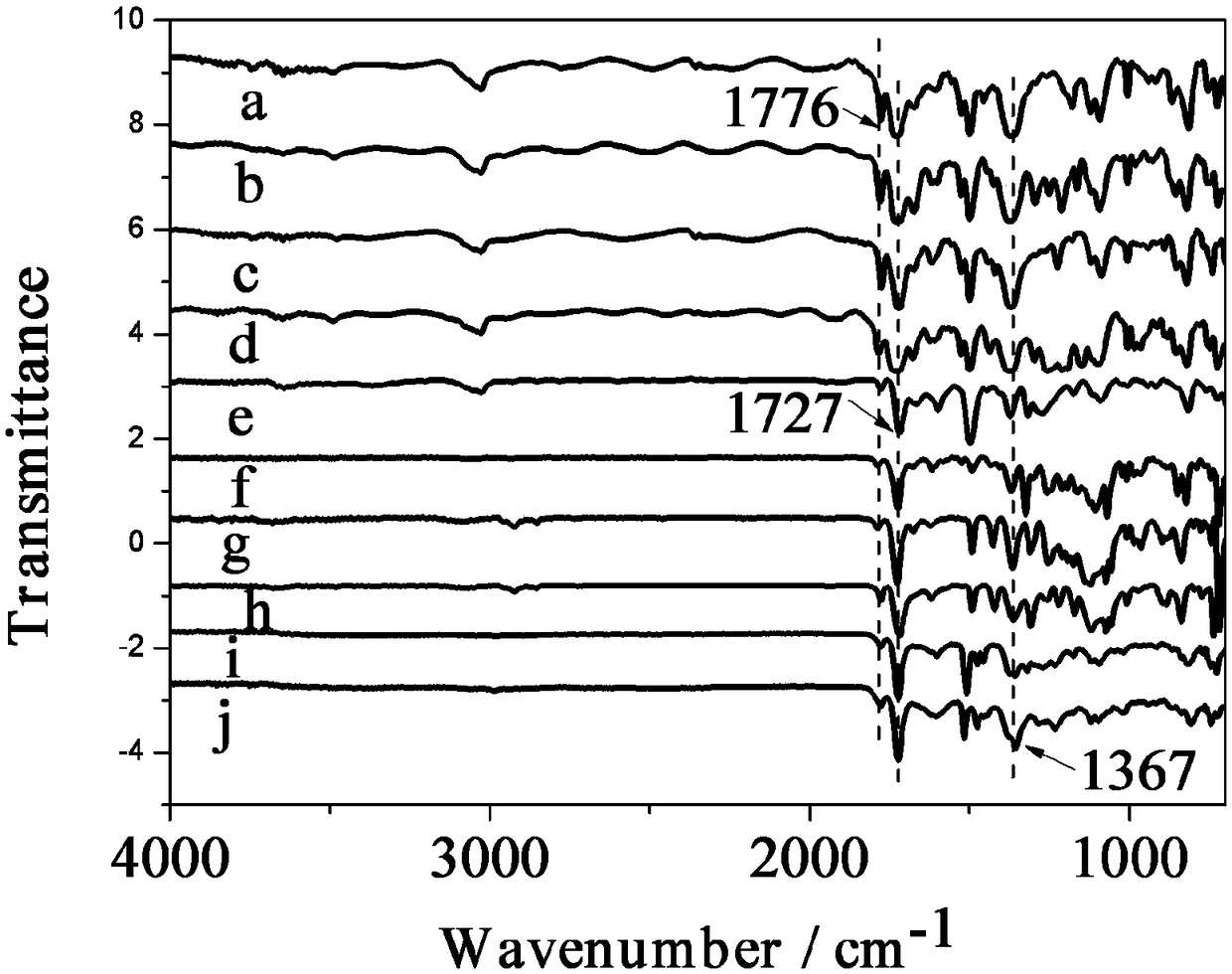
Hyperbranched polyimide containing anthracene structure and preparation method and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
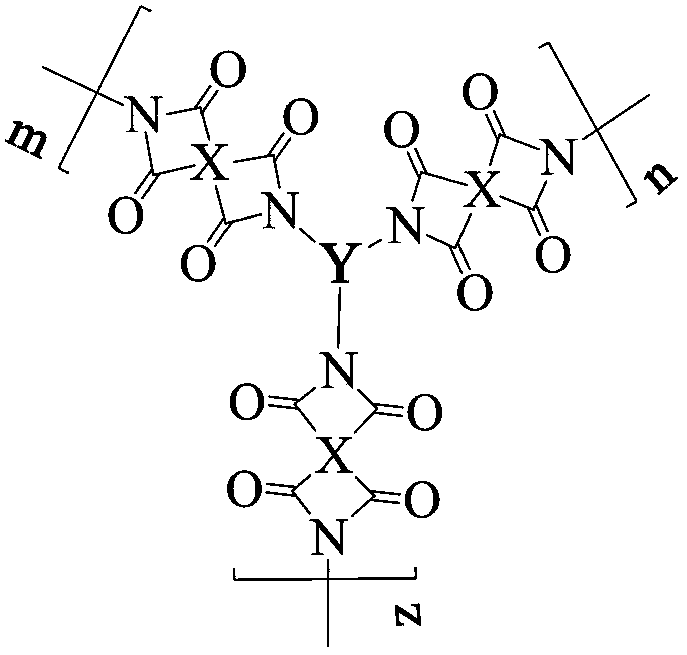
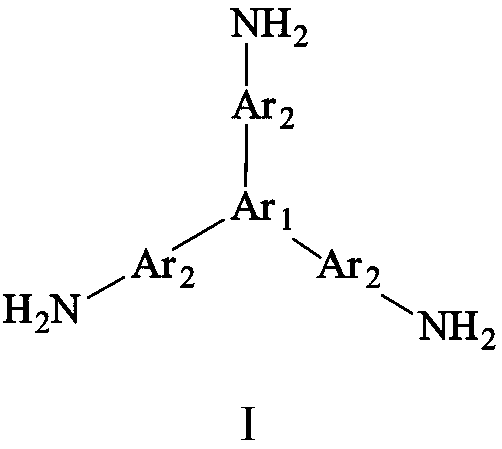
[0040] Add 0.4362g (2mmol) of pyromellitic dianhydride (PMDA) and 36ml of N,N-dimethylformamide into a three-necked flask, blow in argon, raise the temperature to 30°C, and add the triamine monomer 4,4' ,4”-(anthracene-1,9,10-triyl)trianiline 0.4516g (1mmol) was dissolved in 40ml of N,N-dimethylformamide and evenly dropped into the three-necked flask with a constant pressure dropping funnel in 1~2h, Then continue to react for 12 hours, then add 6ml of acetic anhydride and 2ml of triethylamine, heat up to 45°C and continue to react for 10h, after the reaction is completed and cooled to room temperature, discharge the material in ethanol, filter, wash, repeat 2 to 3 times, and finally place in 80 ℃ in a vacuum drying oven for 24 hours to obtain a reddish-brown hyperbranched polyimide polymer, whose structural formula is as follows:
[0041]
Embodiment 2
[0043] Add 0.4515g (2.07mmol) of pyromellitic dianhydride (PMDA) and 15ml of N,N-dimethylacetamide into a three-necked flask, blow in argon, raise the temperature to 30°C, and dilute the triamine monomer 4,4 Dissolve ',4"-(anthracene-2,9,10-triyl)trianiline 0.4516g (1mmol) into 16ml of N,N-dimethylacetamide and drop evenly into the three-necked flask with a constant pressure dropping funnel in 1~2h , then continue to react for 14 hours, then add 6.2ml of acetic anhydride and 2.1ml of triethylamine, heat up to 45°C and continue to react for 14 hours, after the reaction is completed, cool to room temperature and discharge the material in methanol, filter, wash, repeat 2 to 3 times, and finally Place it in a vacuum drying oven at 80°C for 24 hours to obtain a light brown hyperbranched polyimide polymer, whose structural formula is as follows:
[0044]
Embodiment 3
[0046]Add 0.4413g (1.5mmol) of 3,3',4,4'--biphenyltetracarboxylic dianhydride (BPDA) and 10ml of N-methylpyrrolidone into a three-necked flask, pass in argon, raise the temperature to 30°C, and Triamine monomer 5,5',5"-(anthracene-2,6,9-triyl)tris(thiophen-2-amine) 0.4696g (1mmol) dissolved in 8ml N-methylpyrrolidone using a constant pressure dropping funnel Evenly drop into the three-necked flask in 1~2h, then continue to react for 20h, then add 12ml of acetic anhydride and 3ml of triethylamine, heat up to 45°C and continue to react for 10h, after the reaction is completed, cool to room temperature and discharge in methanol, filter and wash , repeated 2 to 3 times, and finally dried in a vacuum oven at 80°C for 24 hours to obtain a brown hyperbranched polyimide polymer, whose structural formula is as follows:
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com