Method for separating sulfuric acid in hydrolyzate of ion exchange resin and collecting and recycling acid
A technology of ion exchange resin and hydrolyzate, applied in the direction of ion exchange, cation exchange, organic cation exchanger, etc., can solve the problems of large discharge of production waste, affecting production operation, troublesome waste water treatment, etc., saving environmental protection treatment costs, The effect of reducing the breaking rate and improving the quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
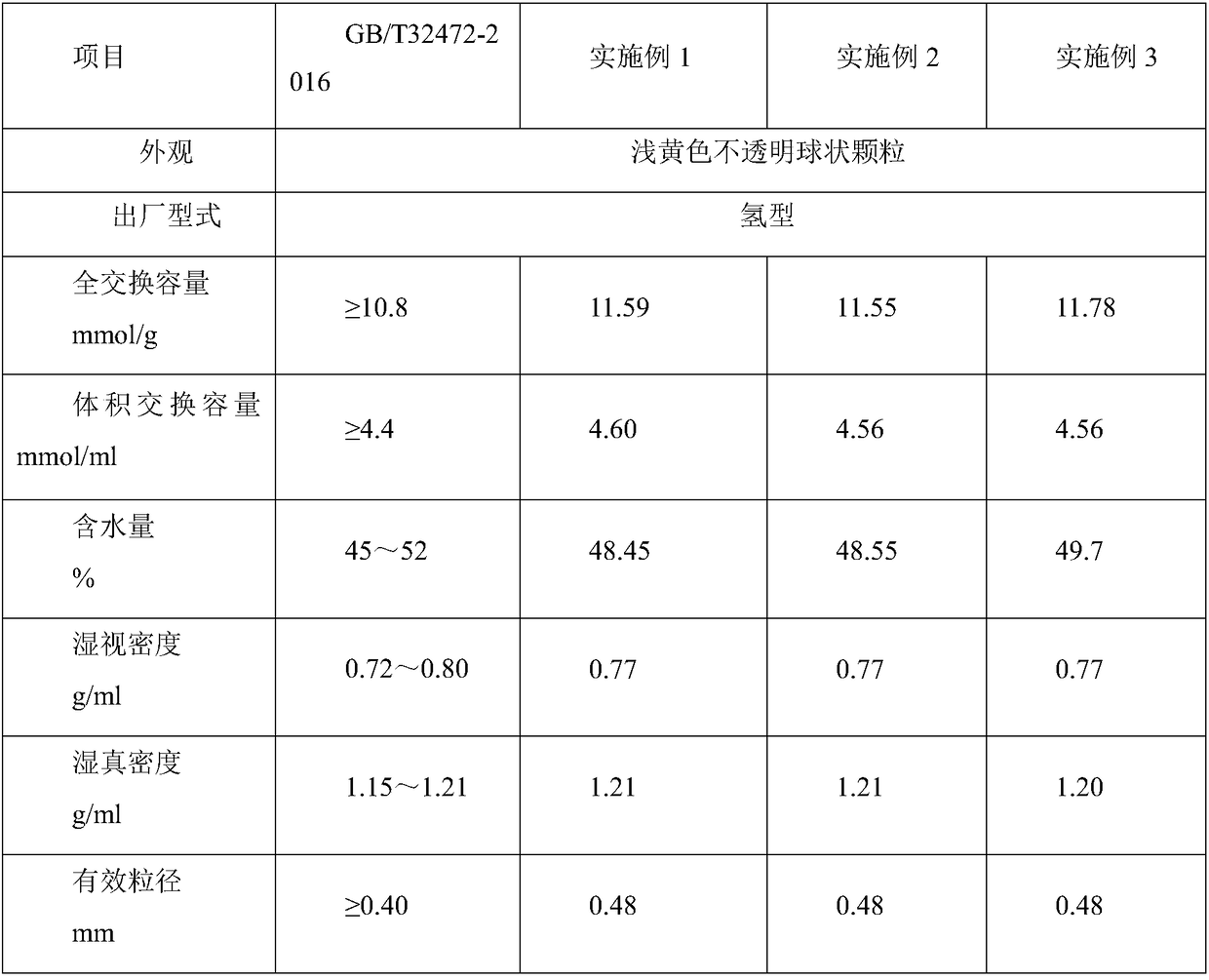
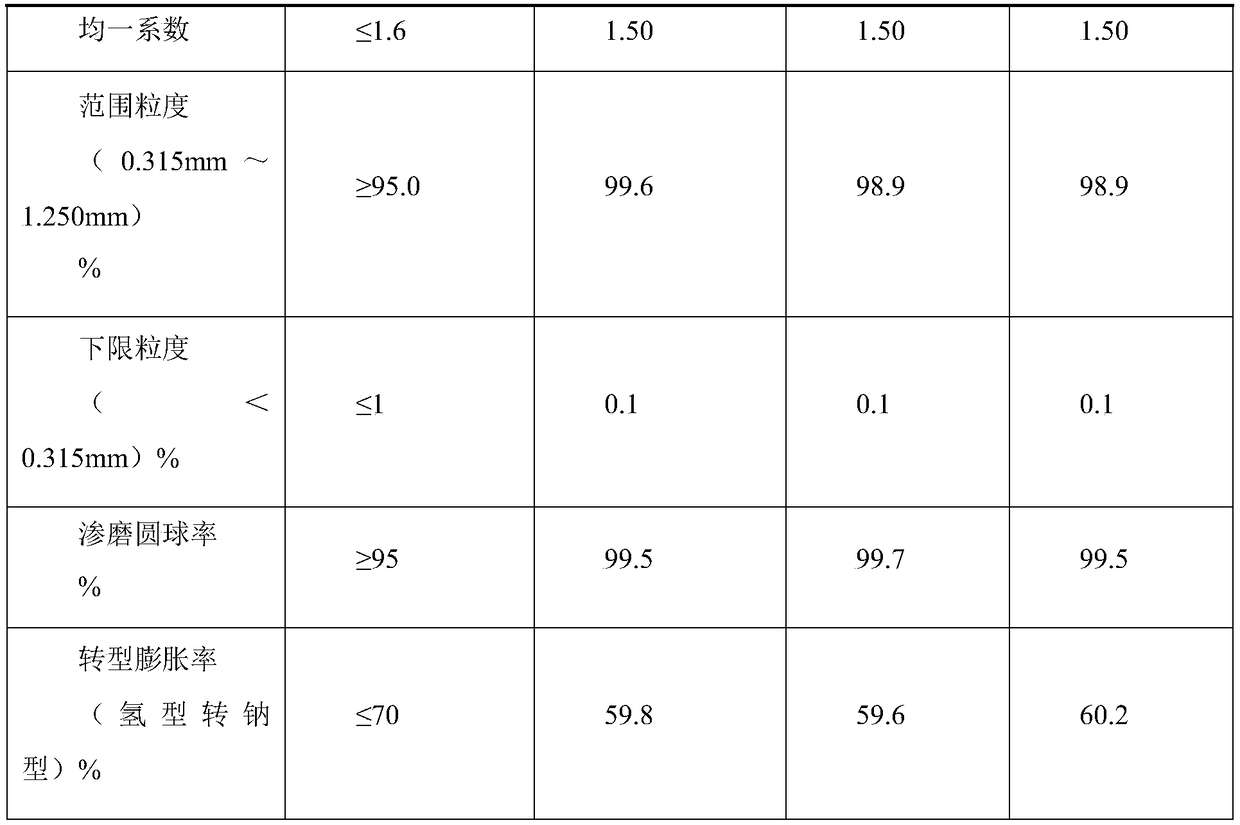
[0028] The macroporous weak acid acrylic ion exchange resin hydrolyzed material is placed in the dilution kettle, filtered through the filter screen into the hydrolyzed liquid collection storage tank, containing sulfuric acid and ammonium sulfate, it is a mixed hydrolyzed liquid, the concentration of sulfuric acid is generally 45~ 48.0%.
[0029] (1) The hydrolyzate in the storage tank was pumped into the reaction kettle with an acid pump, and the chilled brine was turned on for stirring and cooled through the jacket, so that the temperature in the reaction kettle was 5°C, the stirring was stopped, and the crystallization was concentrated for 4h;
[0030] (2) after the lower layer is completely crystallized, the upper layer sulfuric acid liquid is added to the special recovery sulfuric acid header tank with an acid pump, and the purified rear sulfuric acid liquid is for subsequent use;
[0031] (3) the reaction kettle is heated to 70 ℃ through the jacket, after the ammonium su...
Embodiment 2
[0039] The macroporous weak acid acrylic ion exchange resin hydrolyzed material is placed in the dilution kettle, filtered through the filter screen into the hydrolyzed liquid collection storage tank, containing sulfuric acid and ammonium sulfate, it is a mixed hydrolyzed liquid, the concentration of sulfuric acid is generally 45~ 48.0%.
[0040] (1) The hydrolyzate in the storage tank was pumped into the reaction kettle with an acid pump, and the chilled brine was turned on for stirring and cooled through the jacket, so that the temperature in the reaction kettle was 8°C, the stirring was stopped, and the crystallization was concentrated for 5h;
[0041] (2) after the lower layer is completely crystallized, the upper layer sulfuric acid liquid is added to the special recovery sulfuric acid header tank with an acid pump, and the purified rear sulfuric acid liquid is for subsequent use;
[0042] (3) the reaction kettle is heated to 75 ℃ through the jacket, after the ammonium su...
Embodiment 3
[0050] Put the hydrolyzed material of macroporous weakly acidic acrylic ion exchange resin into the dilution tank, and filter it through the filter screen into the hydrolyzate collection storage tank. It contains sulfuric acid and ammonium sulfate, which is a mixed hydrolyzate. The concentration of sulfuric acid is generally 45~ 48.0%.
[0051] (1) Pump the hydrolyzate in the storage tank into the reaction kettle with an acid pump, start stirring, and use frozen brine to cool through the jacket, so that the temperature in the reaction kettle is 10°C, stop stirring, and carry out crystallization and concentration for 6 hours;
[0052] (2) After the lower layer is completely crystallized, add the upper strata sulfuric acid solution into the special recovery sulfuric acid head tank with an acid pump, and obtain the purified sulfuric acid solution for subsequent use;
[0053] (3) The reaction kettle is heated to 80°C through the jacket, and after the ammonium sulfate crystals are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com