Application of deferoxamine to preparation of medicine for preventing and/or treating tumor
A tumor drug, deferoxamine technology, applied in the field of oncology, can solve the problems of adjuvant therapy that have not been reported yet, and achieve the effect of improving the therapeutic effect and enhancing the killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
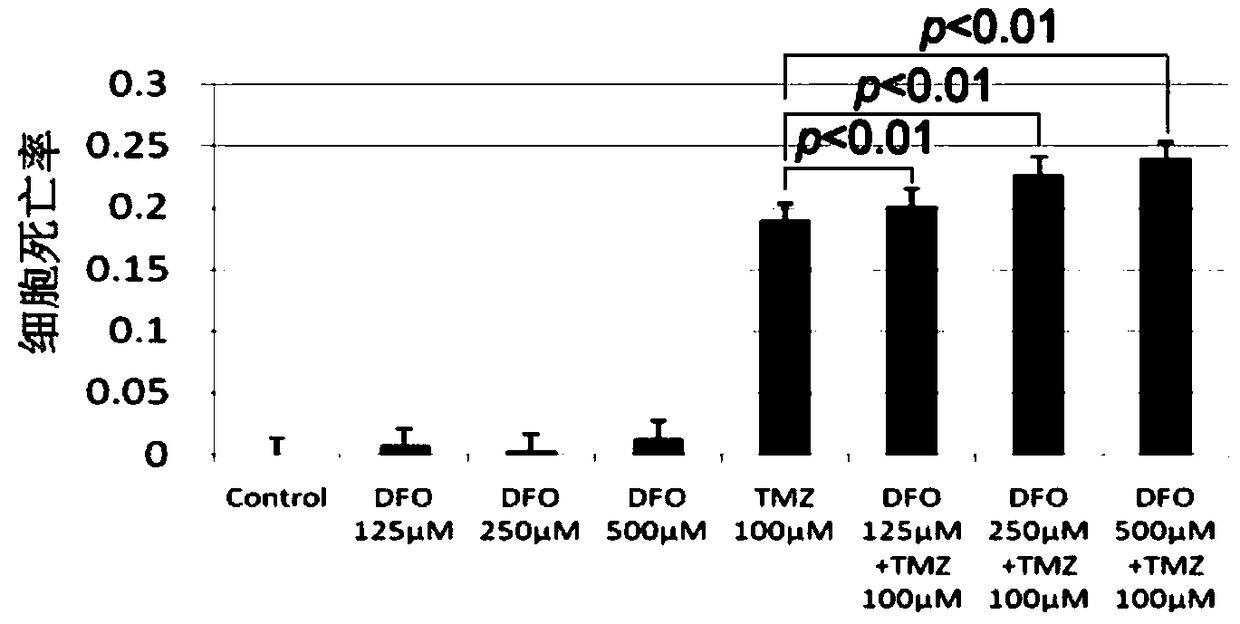
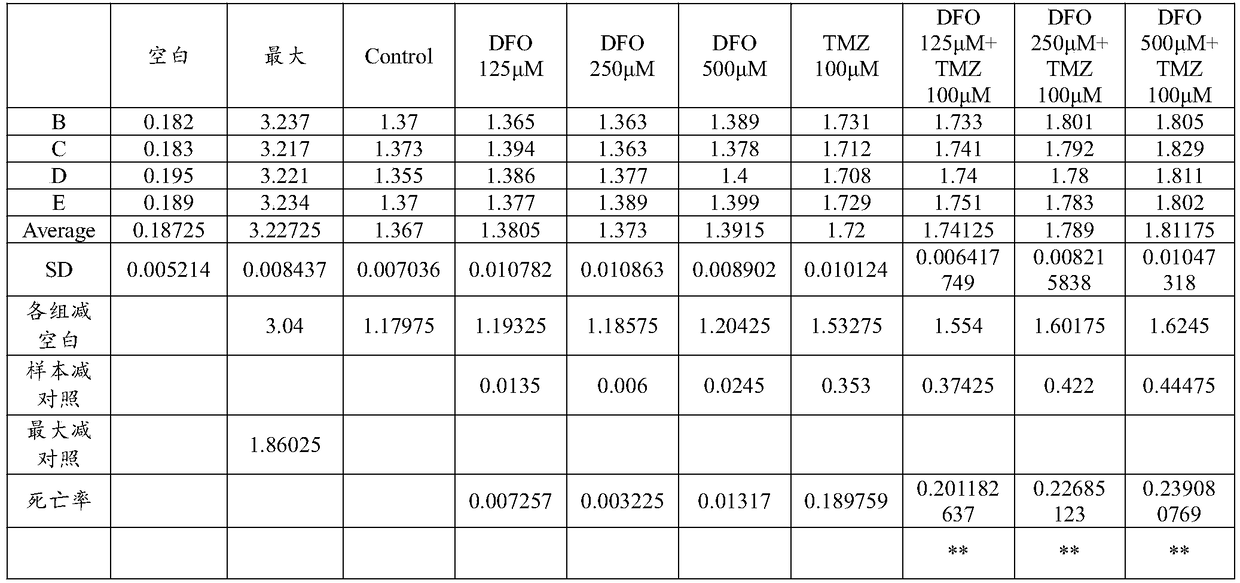
[0031] Example 1 Desferoxamine enhances the therapeutic effect of temozolomide on glioma
[0032] 1. Reagents:
[0033] Desferrioxamine was purchased from Abcam Company, and was prepared to the required concentration with PBS solution when used.
[0034] Temozolomide was purchased from Sigma.
[0035] Human glioma U251 cells were purchased from Shanghai Jikai Biotechnology Company.
[0036] 2. Method:
[0037] (1) The preparation process of different concentrations of deferoxamine is as follows:
[0038] Dissolve deferoxamine in PBS solution to make a mother solution with a concentration of 5 mmol / L. According to the requirements of the experiment, different volumes of mother liquor were added to make the final concentration reach 125 μmol / L, 250 μmol / L, and 500 μmol / L.
[0039] (2) The preparation process of temozolomide with a concentration of 100 μmol / L is as follows:
[0040] Temozolomide was dissolved in DMSO to make a stock solution with a concentration of 50mmol / L...
Embodiment 2
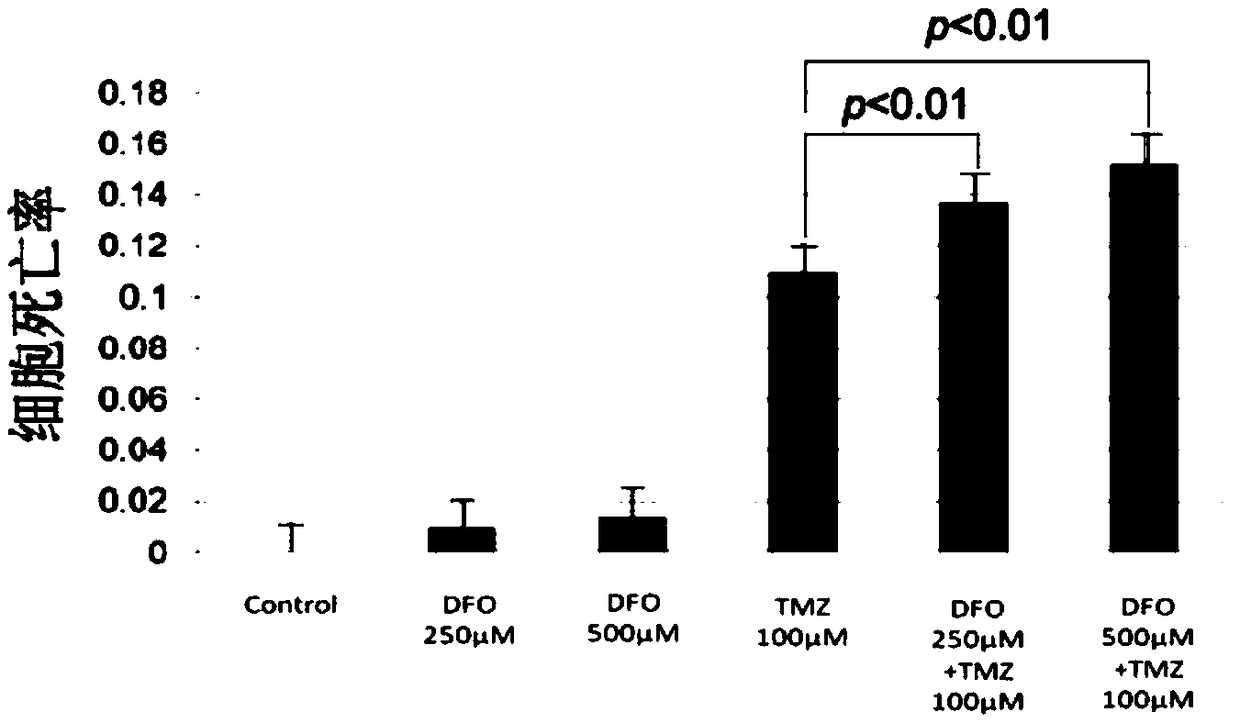
[0046] Example 2 Deferoxamine enhances the therapeutic effect of temozolomide on lung cancer cells
[0047] 1. Reagents:
[0048] Desferrioxamine was purchased from Abcam Company, and was prepared to the required concentration with PBS solution when used.
[0049] Temozolomide was purchased from Sigma.
[0050] Human lung cancer A549 cells were purchased from Shanghai Jikai Biotechnology Company.
[0051] 2. Method:
[0052] (1) The preparation process of different concentrations of deferoxamine is as follows:
[0053] Dissolve deferoxamine in PBS solution to make a mother solution with a concentration of 5 mmol / L. According to the requirements of the experiment, different volumes of mother liquor were added to make the final concentration reach 250 μmol / L and 500 μmol / L.
[0054] (2) The preparation process of temozolomide with a concentration of 100 μmol / L is as follows:
[0055] Temozolomide was dissolved in DMSO to make a mother solution with a concentration of 50 mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com