All-trans retinoic acid quasicrystal and liposome preparation and preparation method thereof
A technology of all-trans retinoic acid and liposome preparations, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., which can solve the problems of unstable release and low drug loading, and achieve the goal of drug delivery. Uniform and stable release, simplified preparation method, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 prepares all-trans retinoic acid liposome
[0055] (1) Weigh 121.742 grams of hydrogenated soybean lecithin (HSPC, molecular weight 783.8), 38.22 grams of distearoylphosphatidylethanolamine-polyethylene glycol 2000 (DSPE-PEG2000) and 40.04 grams of cholesterol, dissolve them with 1.6 milliliters of ethanol, and Water bath in a water bath at 65 degrees Celsius to dissolve and mix to obtain an ethanol mixture;
[0056] (2) Add 6.4 ml of calcium acetate buffer solution (pH 9.0, which consists of 400 mM calcium acetate and water) to the ethanol mixture obtained in step (1), and place it in a water bath at 65 degrees Celsius for 30 minutes to obtain liposome vesicles ;
[0057] (3) The liposome vesicles obtained in step (2) are extruded sequentially through the polycarbonate membrane with a pore size of 200nm, 100nm, 80nm, and 50nm each 8 times, and finally the water phase with an average particle diameter of about 75nm is calcium acetate. of liposomes;
[00...
Embodiment 2~6
[0063] Embodiment 2~6 prepares all-trans retinoic acid liposome
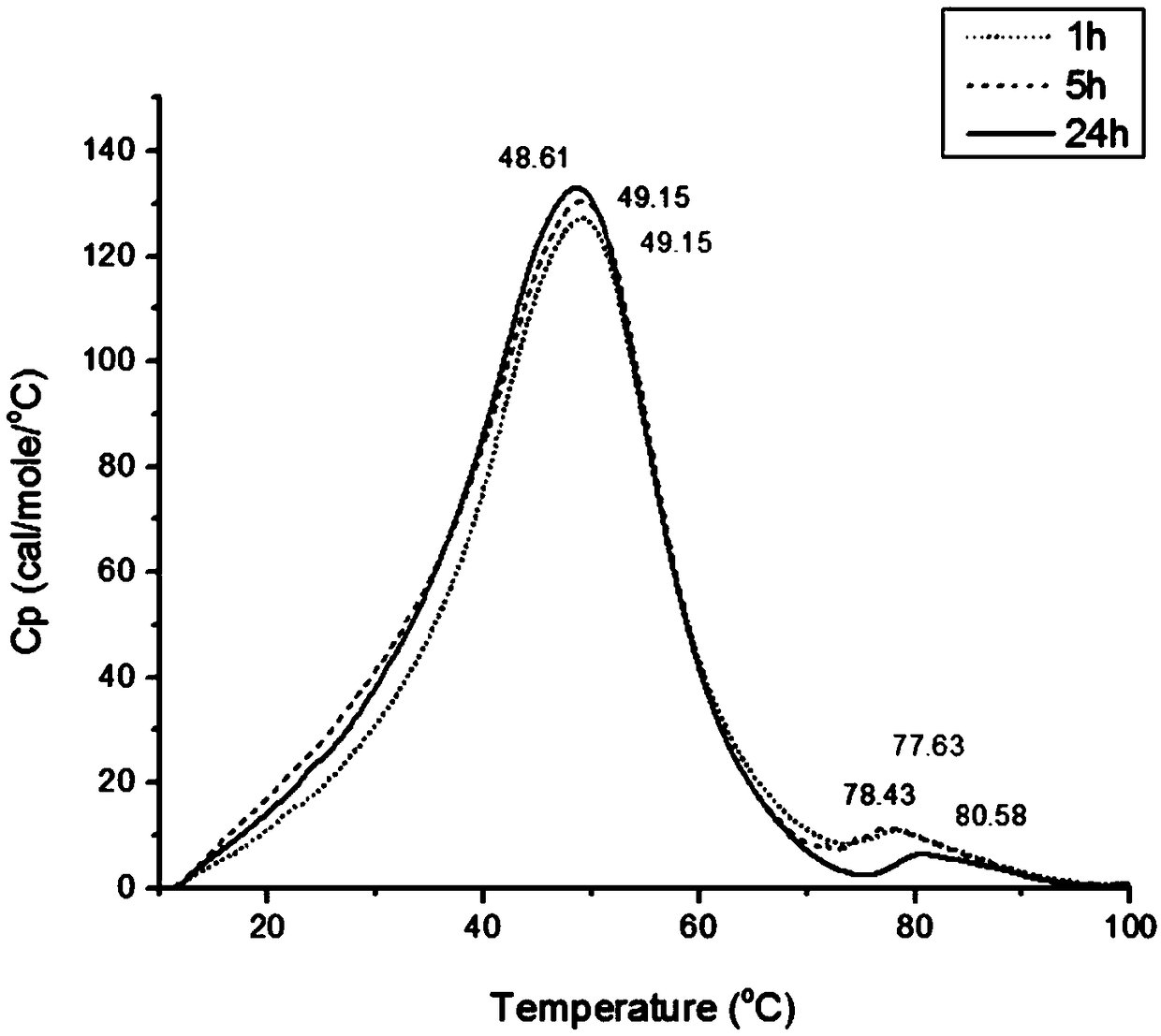
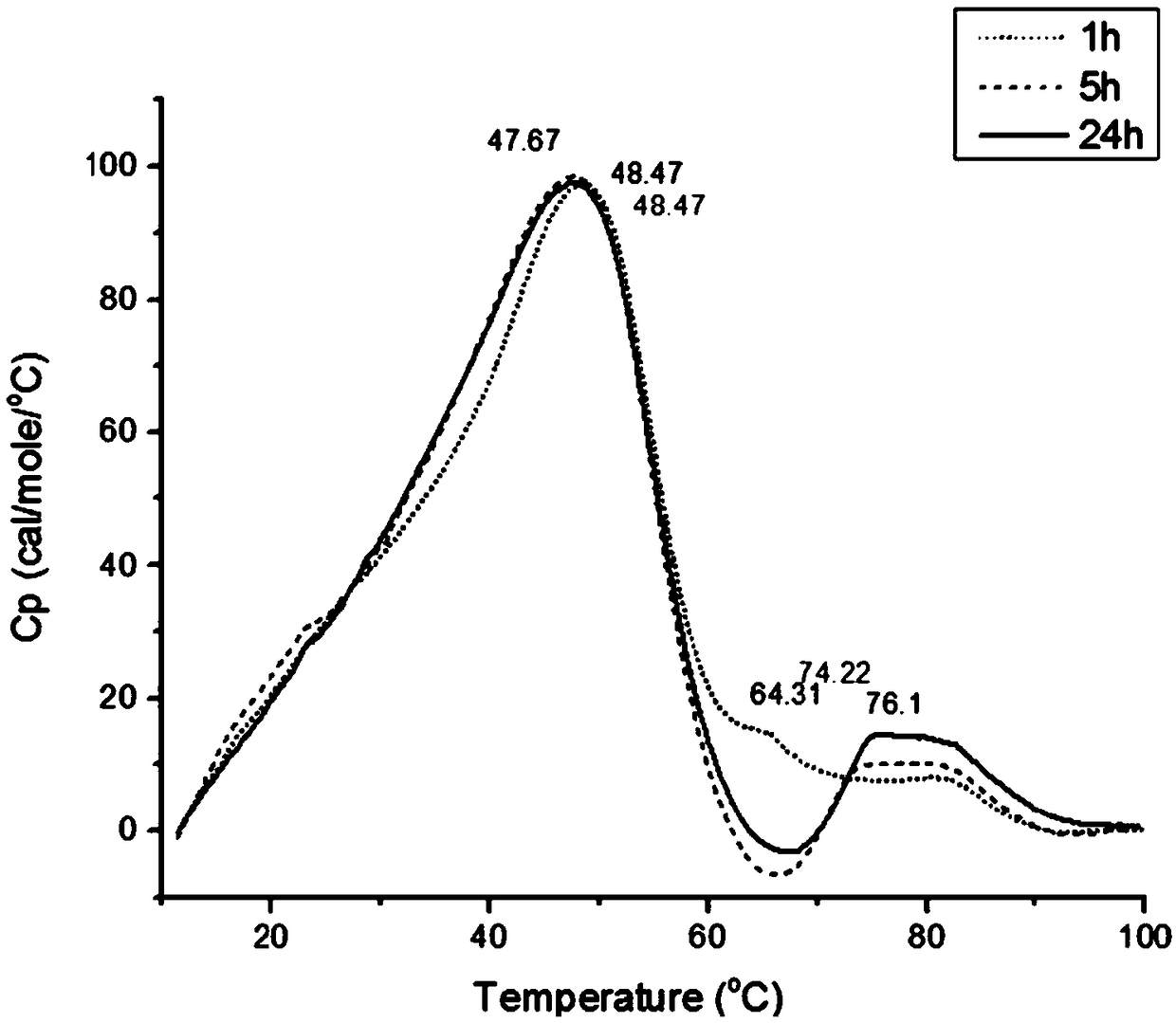
[0064] Using the same method as in Example 1 steps (1), (2), (3), (4), adjust the drug dosage in step (5) and the drug loading time in step (6), and complete Example 2~ 6 and Comparative Examples 1-2 are detailed in the following table.
[0065] Embodiment 2~6 material and result
[0066]
[0067] By comparison, it is found that the all-trans retinoic acid liposome prepared by the preparation method of the present application has a higher drug-to-lipid ratio, and at the same time, the release stability of the drug is also significantly improved with the prolongation of the drug loading time.
Embodiment 7
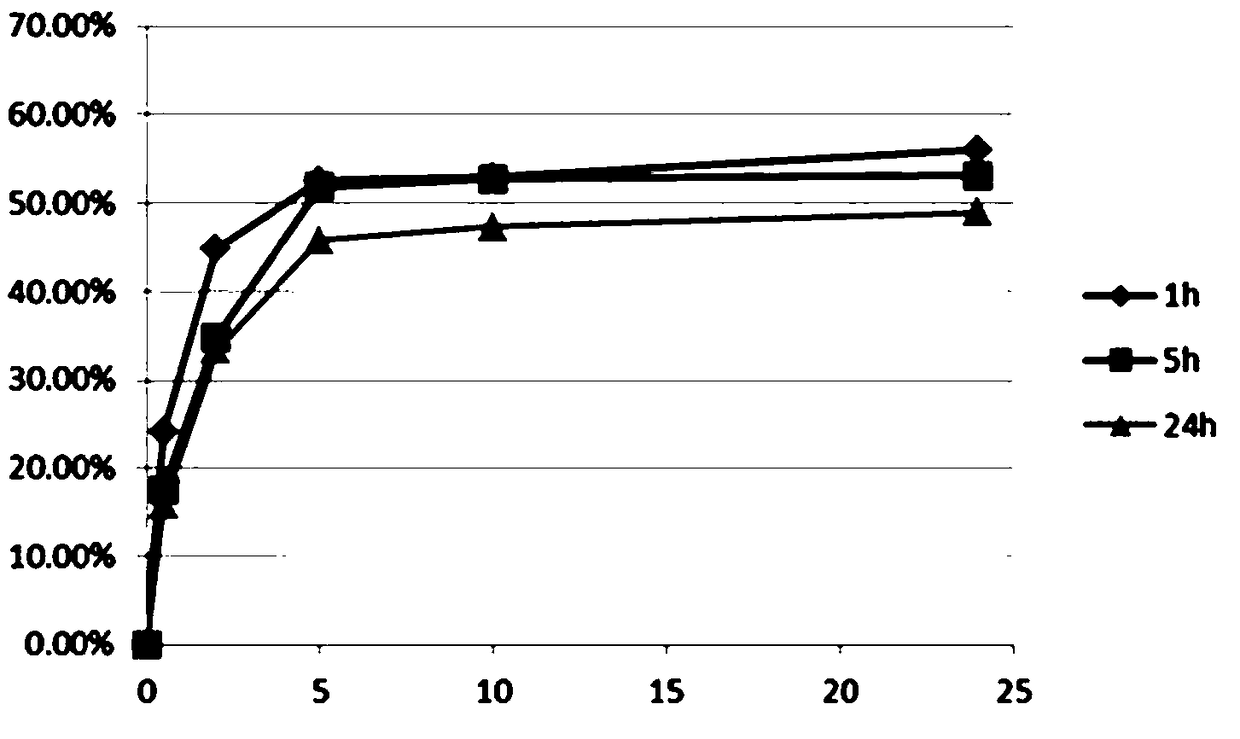
[0075] The all-trans retinoic acid liposome in vitro release situation of embodiment 7 different drug-loading time
[0076] Using 40mg / ml bovine serum albumin (BSA) solution as the release medium, put all-trans retinoic acid liposomes and BSA solution at a ratio of 1:50 (60ul liposomes + 2940ul BSA solution) into a 300KD dialysis bag , 400ml of BSA solution is used as the medium outside the dialysis bag, and the total release system all-trans retinoic acid liposome: BSA solution volume ratio is about 1:6700 times. The protein binding rate of all-trans retinoic acid is 95%. After the release of all-trans retinoic acid drug, it combines with BSA, enters and exits the dialysis bag with BSA, and the drug is taken out of the dialysis bag.
[0077] Select 0.5h, 2h, 5h, 10h, and 24h as sampling points, and measure the unreleased drug content in the dialysis bag each time, and then obtain the in vitro release of all-trans retinoic acid liposomes. In this example, all-trans retinoic a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com