Electroreduction carbon dioxide catalytic material, preparation method and applications thereof
A catalytic material, carbon dioxide technology, applied in the field of electrochemistry, can solve the problems of complex products, inability to produce stable, unstable structures, etc., and achieve the effect of simple synthesis method, abundant raw materials, and excellent catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
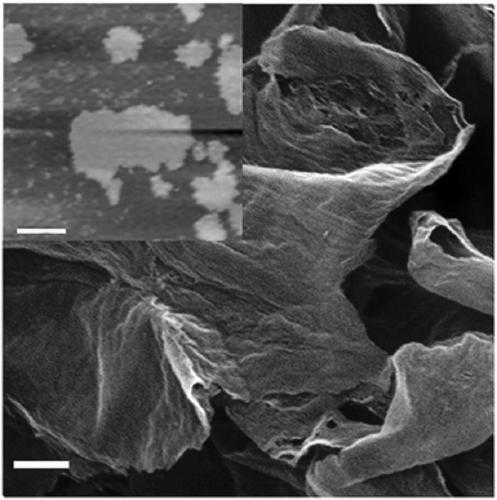
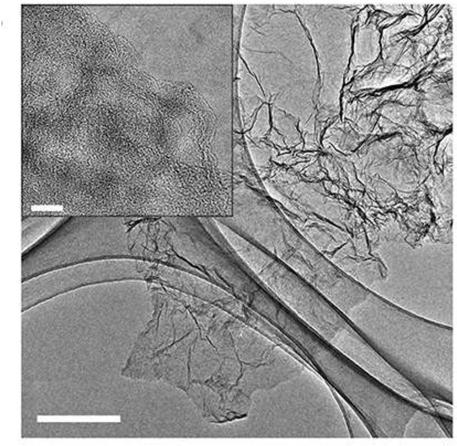
Image
Examples
Embodiment 1
[0047] Preparation of metal (nickel) / carbon catalytic material precursor: Take 24g of melamine, 5g of cysteine, and 0.4g of nickel acetate, put them in a ball mill jar, and then perform ball milling on a ball mill with a ball milling speed of 100rpm / min and a ball milling time of 2h , and fully mixed to obtain the precursor of the catalytic material.
Embodiment 2
[0049] Preparation of metal (nickel) / carbon catalytic material precursor: take 24g melamine, 4.5g glycine, and 0.4g nickel acetate, put them in a ball mill jar, and then perform ball milling on a ball mill with a ball milling speed of 100rpm / min and a ball milling time of 2h. Mix well to obtain the precursor of the catalytic material.
Embodiment 3
[0051] Preparation of metal (nickel) / carbon catalytic material precursor: get 24g melamine, 5g cysteine, 0.4g nickel acetate, 1g conductive carbon material (such as: Ketjen black (KetjenblackEC300J), put in the ball mill jar, then Ball milling is carried out on a ball mill with a ball milling speed of 100 rpm / min and a ball milling time of 2 hours, and the mixture is fully mixed to obtain a carbon-supported catalytic material precursor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com