Method for preparing garnet type Li7La3Zr2O12 electrolyte powder with molten-salt method
A technology of garnet type and electrolyte powder, which is applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., to achieve good effect, simple preparation process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
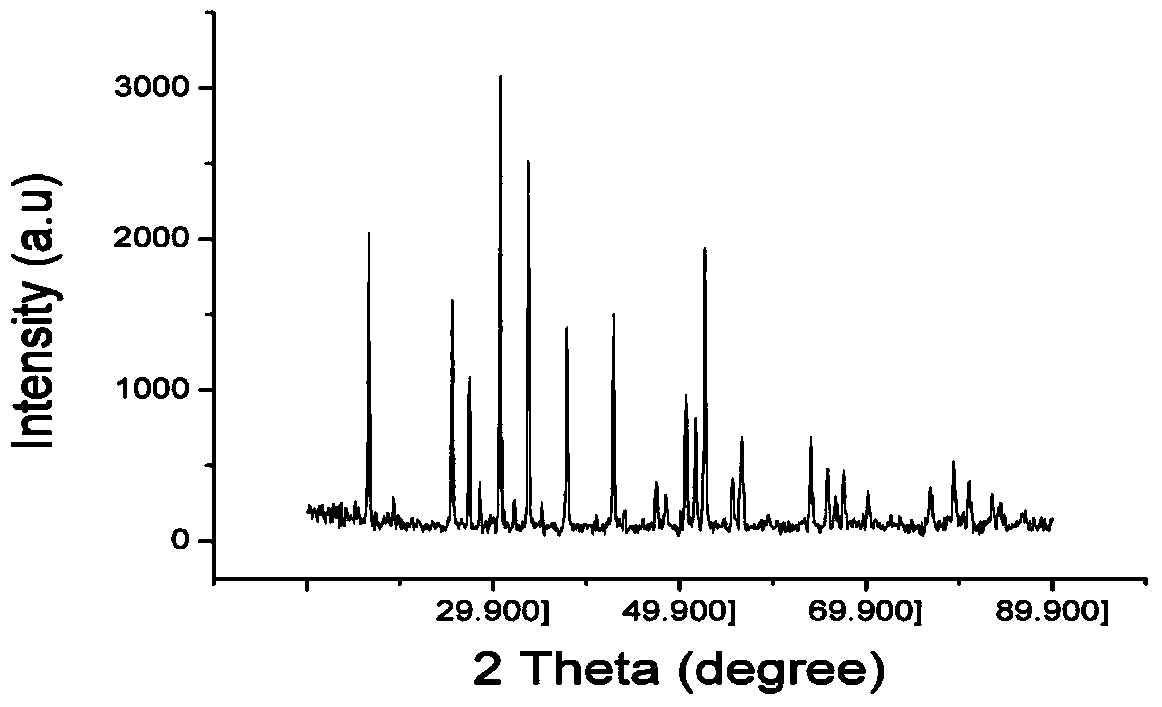
[0027] Drying medicines before weighing them: Li 2 CO 3 Dry at 200°C for 6 hours, La 2 o 3 Dry at 900°C for 24 hours, weigh Li according to the stoichiometric ratio of Li:La:Zr=7:3:2 2 CO 3 , La 2 o 3 , ZrO 2, in order to compensate for the loss of lithium in the calcination process, lithium carbonate was increased by 10% according to the mass fraction. Weigh KCl and LiCl according to the KCl:LiCl molar ratio of 58:42. According to Li 2 CO 3 , La 2 o 3 , ZrO 2 The mass ratio of mixture to molten salt is 1:4 to prepare mixed powder. The mixed powder was poured into a zirconia ball mill jar, and ball milled on a planetary ball mill for 10 hours. The ball-milled and mixed powder was dried at 100°C for 10 hours, and packed into a ziplock bag. Take an appropriate amount of mixed powder and place it in an alumina crucible, set the temperature of the electric furnace to 1100°C, place the mixed powder in the electric furnace for 8 hours, cool to room temperature natural...
Embodiment 2
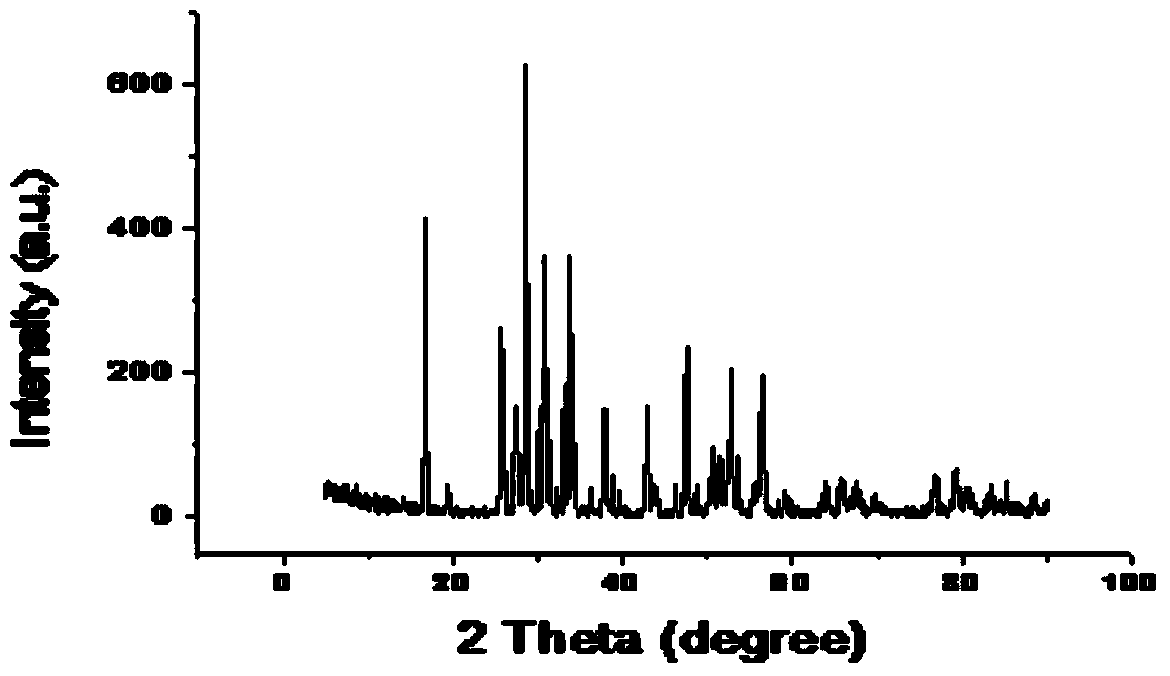
[0030] Drying medicines before weighing them: Li 2 CO 3 Dry at 200°C for 6 hours, La 2 o 3 Dry at 900°C for 24 hours, weigh Li according to the stoichiometric ratio of Li:La:Zr=7:3:2 2 CO 3 , La 2 o 3 , ZrO 2 , in order to compensate for the loss of lithium in the calcination process, lithium carbonate was increased by 10% according to the mass fraction. Weigh KCl and LiCl according to the KCl:LiCl molar ratio of 58:42. According to Li 2 CO 3 , La 2 o 3 , ZrO 2 The mass ratio of mixture to molten salt is 1:4 to prepare mixed powder. The mixed powder was poured into a zirconia ball mill jar, and ball milled on a planetary ball mill for 10 hours. The ball-milled and mixed powder was dried at 100°C for 10 hours, and packed into a ziplock bag. Take an appropriate amount of mixed powder and place it in an alumina crucible, set the temperature of the electric furnace to 900°C, place the mixed powder in the electric furnace for 6 hours, cool it to room temperature natu...
Embodiment 3
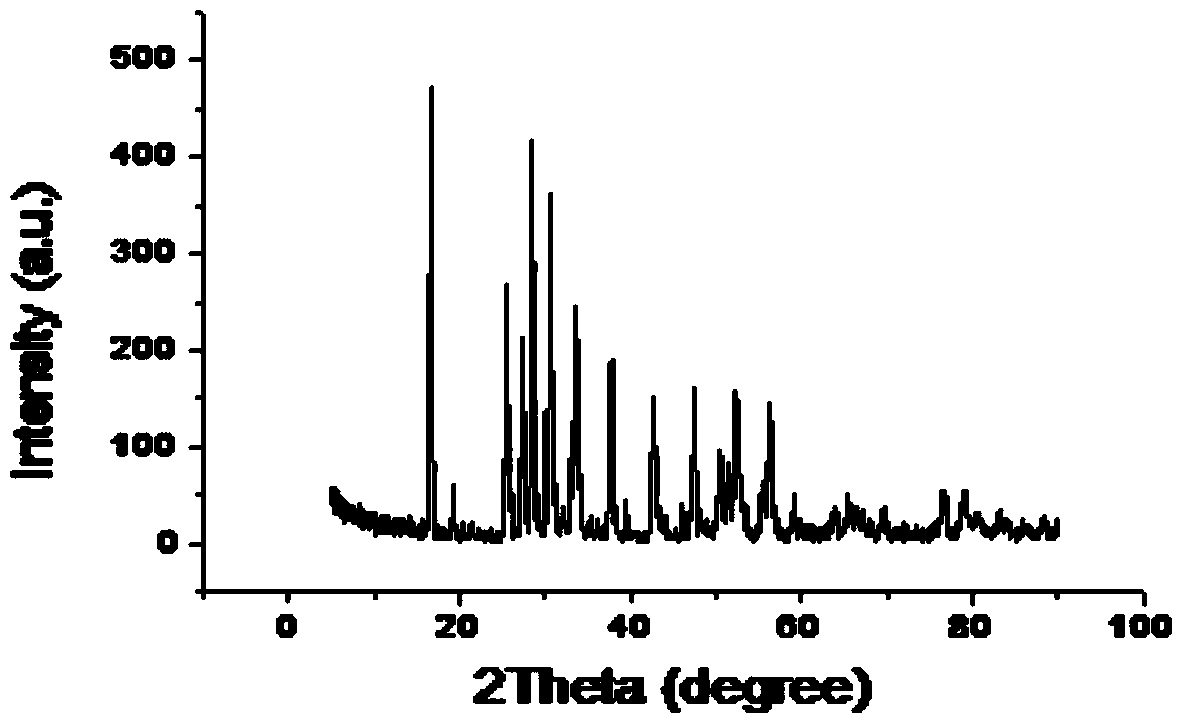
[0033] Drying medicines before weighing them: Li 2 CO 3 Dry at 200°C for 6 hours, La 2 o 3 Dry at 900°C for 24 hours, weigh Li according to the stoichiometric ratio of Li:La:Zr=7:3:2 2 CO 3 , La 2 o 3 , ZrO 2 , in order to compensate for the loss of lithium in the calcination process, lithium carbonate was increased by 10% according to the mass fraction. Weigh KCl and LiCl according to the KCl:LiCl molar ratio of 58:42. According to Li 2 CO 3 , La 2 o 3 , ZrO 2 The mass ratio of mixture to molten salt is 1:4 to prepare mixed powder. The mixed powder was poured into a zirconia ball mill jar, and ball milled on a planetary ball mill for 10 hours. The ball-milled and mixed powder was dried at 100°C for 10 hours, and packed into a ziplock bag. Take an appropriate amount of mixed powder and place it in an alumina crucible, set the temperature of the electric furnace to 900°C, place the mixed powder in the electric furnace for calcination for 4 hours, cool it to room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com